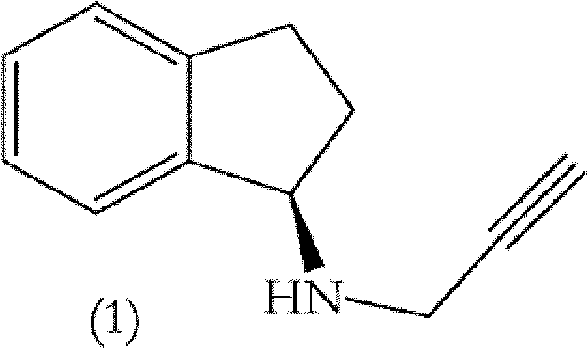
Polymorphic form of rasagiline mesylate
A technology of rasagiline mesylate and mesylate, which is applied in the field of polymorphic forms of rasagiline mesylate, and can solve the problems of undisclosed issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
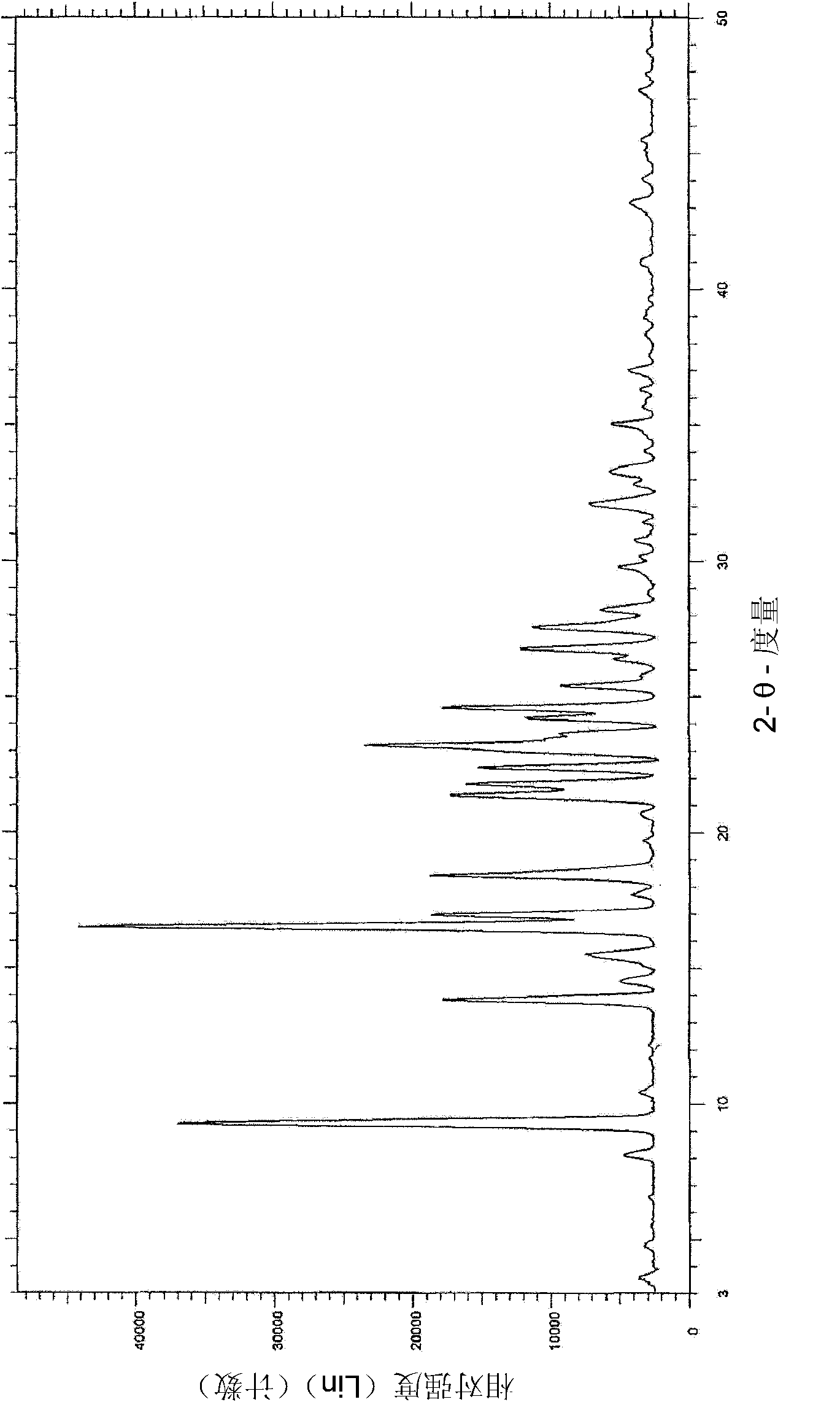
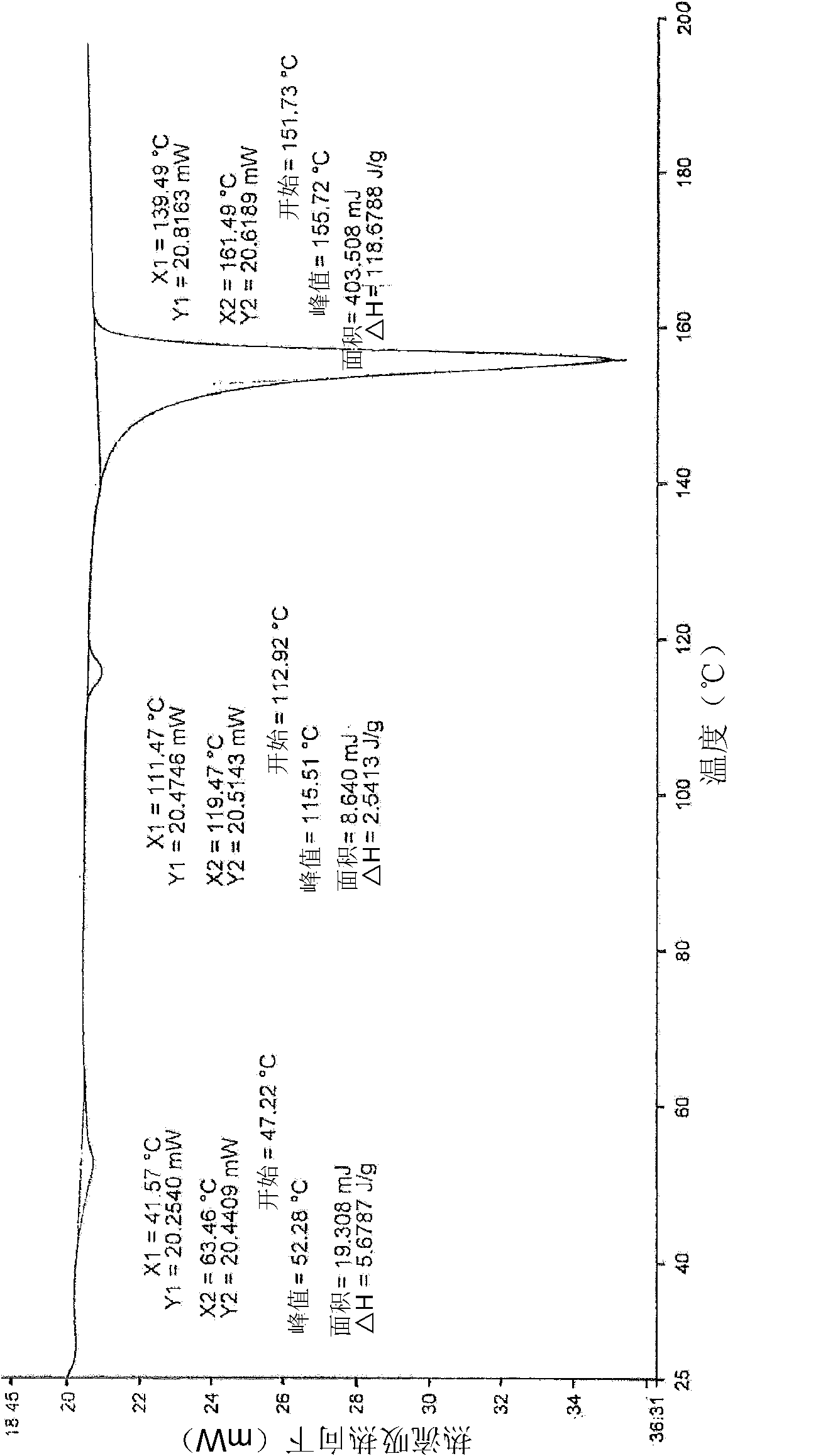
[0049] Rasagiline mesylate (1 eq) was placed in acetonitrile (4 vol) and heated to 55°C for 20-30 minutes until a clear solution was obtained. The solution was cooled to 25°C for 30 minutes and filtered. The solid product was dried under vacuum at 25-30°C for 2 hours. XRPD and DSC analysis data confirmed that the obtained product was a crystalline form of rasagiline mesylate.
Embodiment 2
[0051] Rasagiline mesylate (1 eq) was placed in THF (20 vol) and heated to 66°C for 20-30 minutes. Obtain a slurry. The slurry was cooled to 25°C for 30 minutes and filtered. The solid product was dried under vacuum at 25-30°C for 2 hours. XRPD and DSC analysis data confirmed that the obtained product was a crystalline form of rasagiline mesylate.
Embodiment 3
[0053] Rasagiline mesylate (1 eq) was dissolved in 1-butanol (3 vol) and heated to 55°C for 20-30 minutes until a clear solution was obtained. The solution was cooled to 25°C for 30 minutes and filtered. The solid product was dried under vacuum at 25-30°C for 2 hours. XRPD and DSC analysis data confirmed that the obtained product was a crystalline form of rasagiline mesylate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com