Impurity preparation and analysis method of rasagiline mesylate
A technology of rasagiline mesylate and a detection method, applied in the fields of analytical chemistry and pharmaceutical analytical chemistry, to achieve the effects of high purity, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
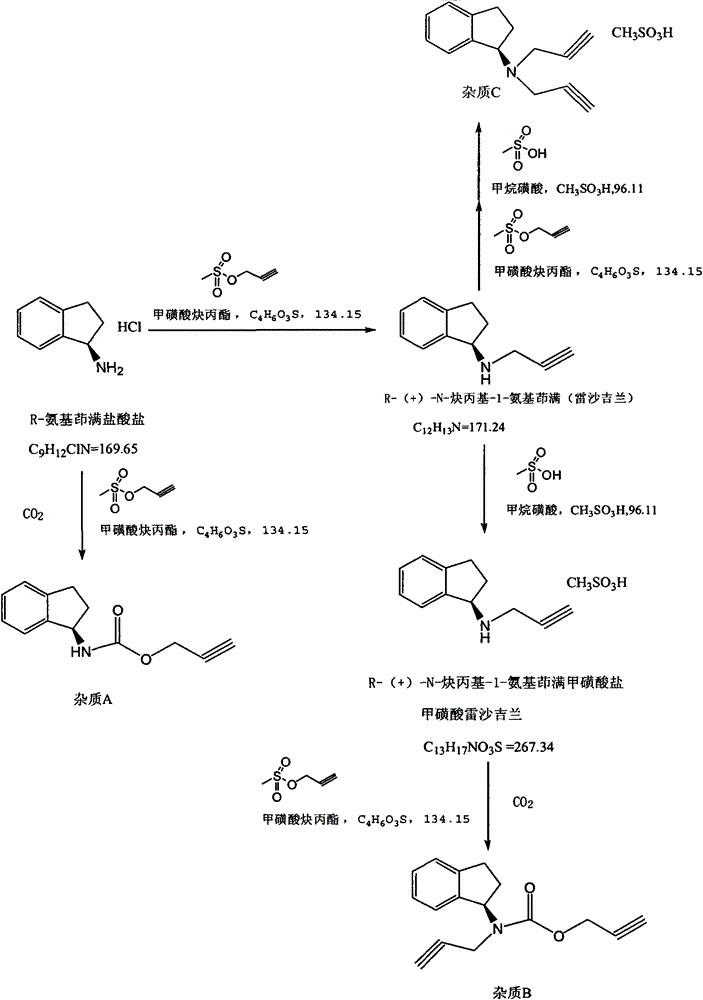
[0114] Embodiment 1: the preparation of rasagiline mesylate
[0115] ① Dissolve 133g of R-aminoindane in a reaction flask with dimethylformamide, stir evenly, add 138g of potassium carbonate, stir, and add dropwise a DMF solution of 96.1g of propargyl methanesulfonate. Process control temperature 25-35°C, stirring and reacting for more than 20 hours; cooling, adding water, extracting the water phase twice with dichloromethane, combining dichloromethane, washing with acid water, separating the water phase, adding ethyl acetate and oxidizing with hydroxide Wash with sodium solution, extract twice with ethyl acetate, dry, filter, and concentrate under reduced pressure to obtain about 112 g of viscous liquid (crude rasagiline).
[0116] ②Dissolve the viscous liquid obtained above in 560mL of isopropanol, heat above 60°C, add methanesulfonic acid to adjust the pH under stirring, the pH is ≤4 after the addition, reflux for about 20 minutes, cool to crystallize, filter, and dry under...
Embodiment 2
[0117] Embodiment 2: the separation of impurity A, impurity B
[0118] Concentrate the dichloromethane solution after washing with acid water in Step ① of Example 1 to dryness to obtain a solid, which can be purified and treated by high-pressure liquid phase preparation to obtain a high-purity impurity reference substance. The specific operations are as follows:
[0119] Purification conditions:
[0120]
[0121] Gradient elution ratio:
[0122] time (minutes)
A%
B%
0
80
20
30
50
50
60
50
50
[0123] Specific steps:
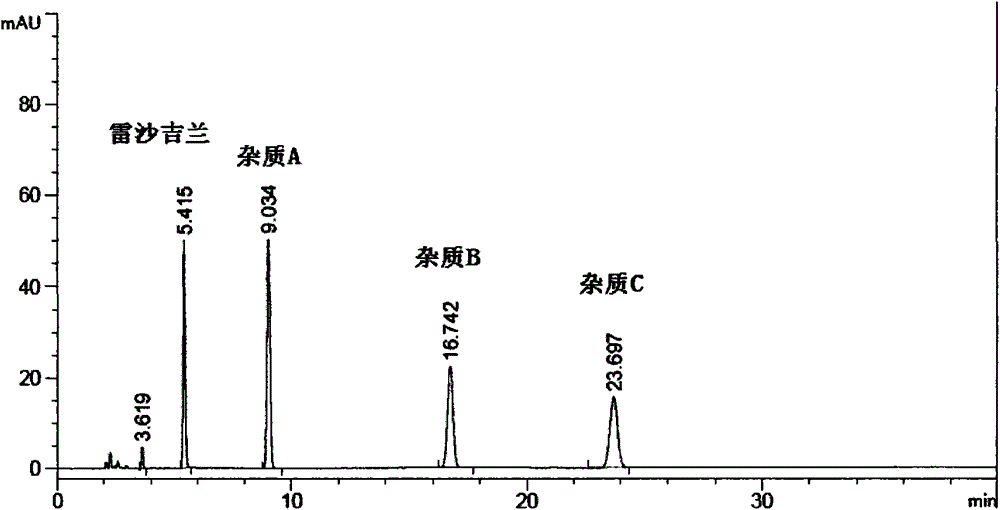
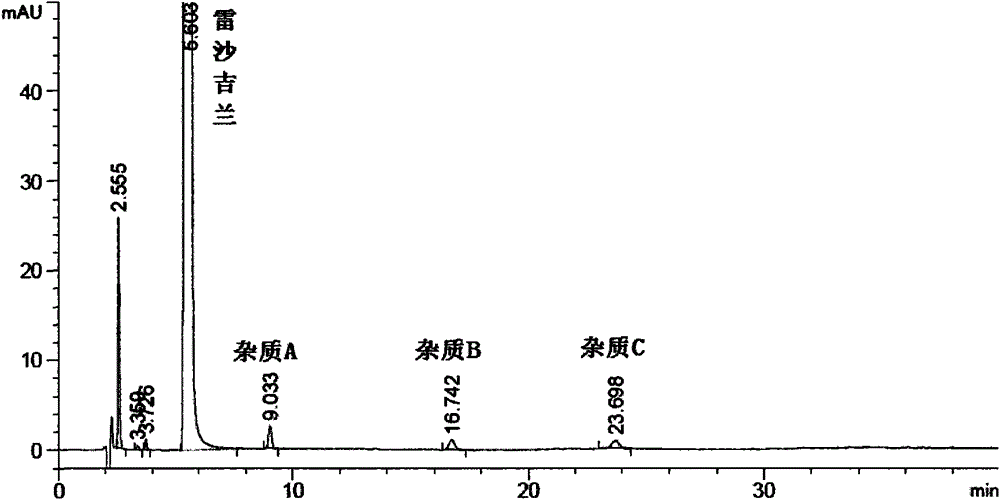
[0124] Concentrate the dichloromethane solution washed with acid water in Example 1 to dryness to obtain a solid, take 1g, dissolve it with 50% acetonitrile / water, filter, put it on the column, and elute it with a gradient program. According to the peak situation, collect it in sections For the eluate, the eluate containing impurity A and impurity B with a purity >98% were collected and combined respec...
Embodiment 3
[0148] Embodiment 3: the separation of impurity C
[0149] Concentrate and dry the isopropanol filtrate obtained in step ② of Example 1 to obtain a solid, which can be purified and treated by high-pressure liquid phase preparation to obtain a high-purity impurity C reference substance. The specific operations are as follows:
[0150] Purification conditions:
[0151]
[0152] Gradient elution ratio:
[0153] time (minutes)
A%
B%
0
80
20
30
50
50
60
50
50
[0154] Specific steps:
[0155] Take the isopropanol filtrate obtained in step ② of Example 1 and concentrate to dry 1g of the solid obtained, dissolve it with 20% acetonitrile / water, put it on the column, and gradient elution, according to the peak situation, collect the eluent in sections, with a purity of >95% The eluents were combined. Concentrate and evaporate to dryness to obtain a white or off-white solid (impurity C), add 3 times the amount of ethanol to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com