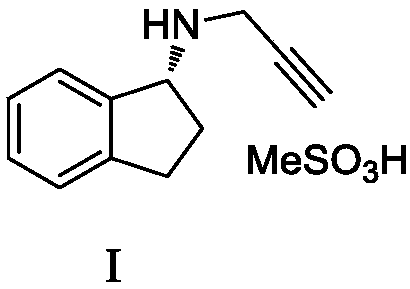
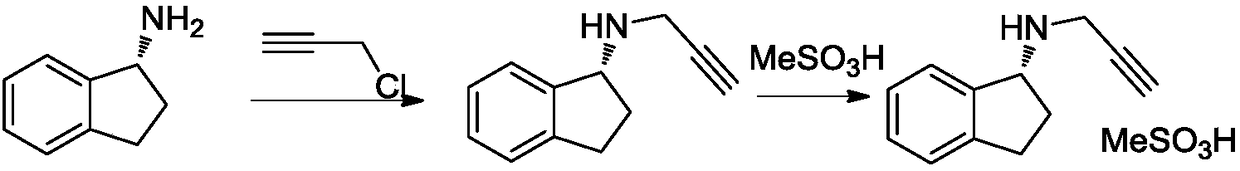
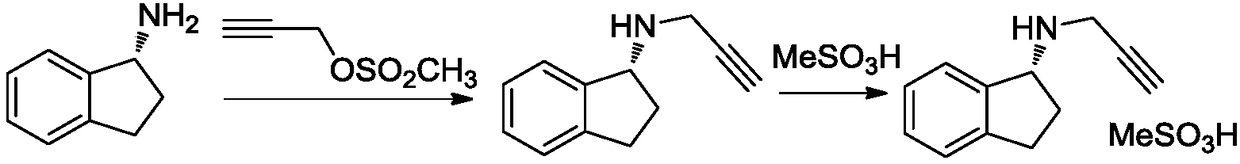
Preparation method of rasagiline mesylate and intermediate thereof
A technology of rasagiline mesylate and intermediates, which is applied in the field of preparation of rasagiline mesylate and intermediates thereof, can solve the problems of many side reactions, low reaction yield and high impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1: the preparation of rasagiline intermediate II
[0067]
[0068] Under nitrogen protection, add (R)-(-)-1-aminoindane 22g (0.165mol) and propiolic acid 16.2g (0.231mol) into 440mL of dichloromethane, and add dicyclohexylcarbodiethylene 57.8g (0.280mol) of amine and 36.3g (0.297mol) of 4-dimethylaminopyridine were stirred at 15-20°C for 3-4 hours. A 12% hydrochloric acid aqueous solution was added to quench (the stated mass concentration refers to the percentage of the mass of hydrogen chloride in the total mass of the hydrochloric acid aqueous solution), and then extracted with dichloromethane. The mass concentration of the organic phase is 10% aqueous sodium hydroxide solution (the mass concentration refers to the percentage of the quality of sodium hydroxide in the total mass of the aqueous sodium hydroxide solution), and the mass concentration is 10% aqueous sodium bicarbonate solution (the mass concentration of the Concentration refers to that the q...
Embodiment 2
[0069] Embodiment 2: the preparation of rasagiline intermediate II
[0070] Under the protection of nitrogen, add 33g (0.248mol) of (R)-(-)-1-aminoindane and 22.1g (0.315mol) of propiolic acid to 495mL of tetrahydrofuran, and add 1-hydroxybenzotriazole 42.7g under stirring. g (0.316 mol), 62.7 g (0.327 mol) of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, and then 37.6 g (0.621 mol) of triethylamine were added. Stir at 20-25°C for 2-3 hours. A 12% hydrochloric acid aqueous solution was added to quench (the stated mass concentration refers to the percentage of the mass of hydrogen chloride in the total mass of the hydrochloric acid aqueous solution), and then extracted with dichloromethane. The mass concentration of the organic phase is 10% aqueous sodium hydroxide solution (the mass concentration refers to the percentage of the quality of sodium hydroxide in the total mass of the aqueous sodium hydroxide solution), and the mass concentration is 10% aqueous sodium...
Embodiment 3
[0071] Embodiment 3: the preparation of rasagiline intermediate II
[0072] Under nitrogen protection, add (R)-(-)-1-aminoindan 22g (0.165mol) and propiolic acid 12.7g (0.181mol) to 220mL of 2-methyltetrahydrofuran, and add O-(7 -Azabenzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate 68.9g (0.181mol), then add diisopropylethylamine 31.9g ( 0.247mol). Stir at 10-15°C for 1-2 hours. A 12% hydrochloric acid aqueous solution was added to quench (the stated mass concentration refers to the percentage of the mass of hydrogen chloride in the total mass of the hydrochloric acid aqueous solution), and then extracted with dichloromethane. The mass concentration of the organic phase is 10% aqueous sodium hydroxide solution (the mass concentration refers to the percentage of the quality of sodium hydroxide in the total mass of the aqueous sodium hydroxide solution), and the mass concentration is 10% aqueous sodium bicarbonate solution (the mass concentration described is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com