Application of rhodamine 6G ramifications
A technology of derivatives and compounds, applied in the fields of rhodamine 6G derivatives and cell lysosomal fluorescent dyes, can solve the problems of short retention period, loss of probes, loss of fluorescence performance, etc., and achieve high photostability and retention time long effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] 1) Add rhodamine 6G (2g) into a flask containing 20mL of ethylenediamine, stir the system electromagnetically, react at 80°C for 12 hours, remove unreacted solvent, separate and purify through a silica gel column to obtain rhodamine 6G-ethylenediamine Amines, whose chemical structural formula is:
[0027]
[0028] R=-NH 2 ,-N(CH 3 ) 2 ,-CH 2 -N(CH 3 ) 2 .
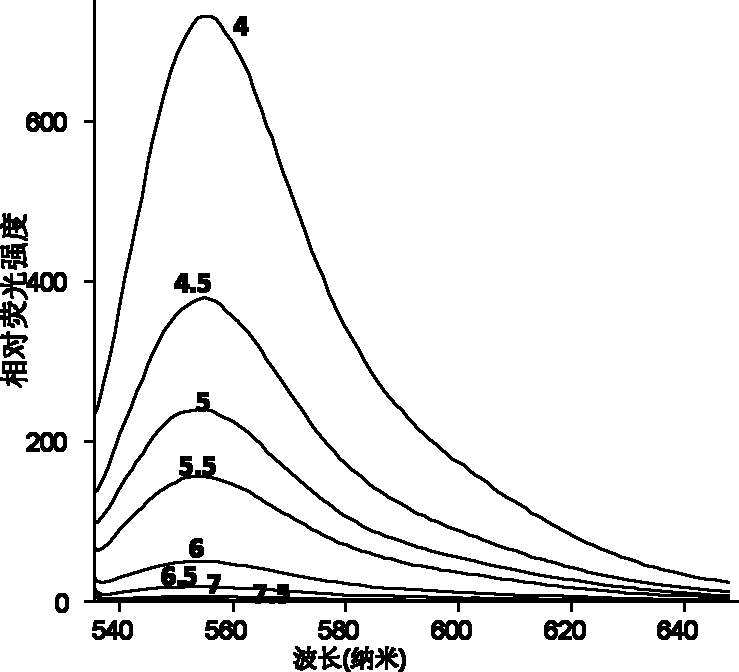
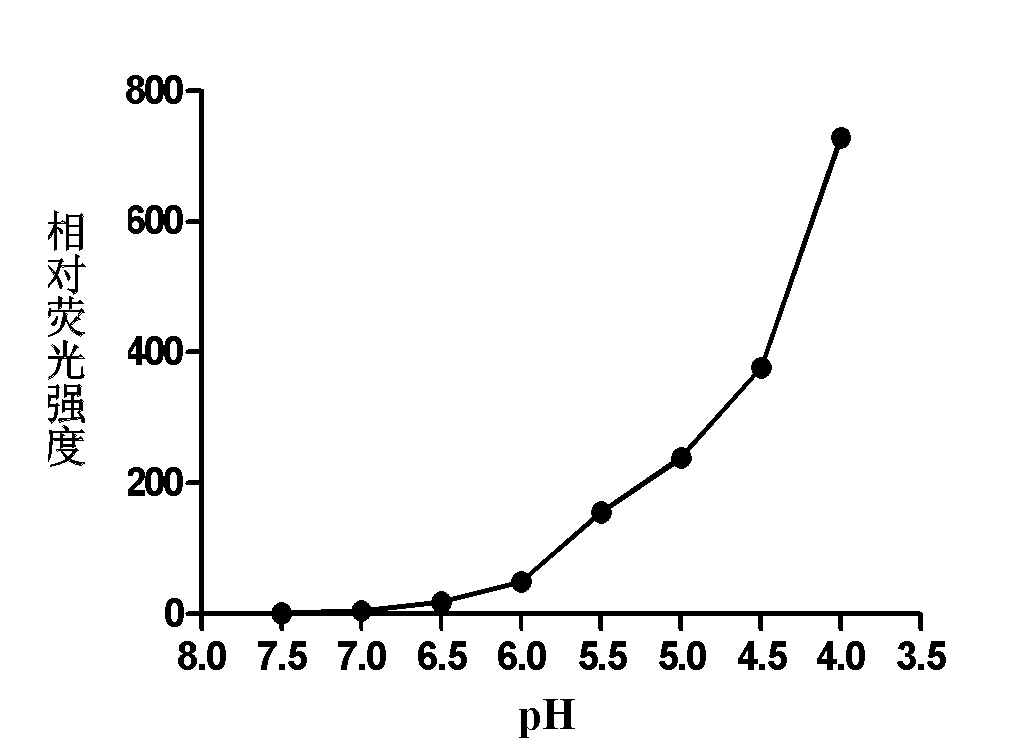
[0029] 2) Detection of the fluorescence emission intensity of rhodamine 6G-ethylenediamine (1 μg / mL) containing a 5-membered ring in the molecule in buffer solutions of different pH values (7.0, 6.5, 6.0, 5.5, 5.0, 4.5, 4.0) . It was found that under the conditions of pH4.0 and 7.0, the fluorescence intensity of rhodamine 6G-ethylenediamine was increased by 1000 times (Ex: 530 / Em 560nm) (see figure 1 with 2 ), the acid of rhodamine 6G-ethylenediamine derivative in embodiment 1 initiates and produces fluorescent chemical reaction route as follows:
[0030]
[0031]3) Rhodamine 6G-ethylenediamine (10 ...
Embodiment 2
[0036] Embodiment 2: Rhodamine 6G-N, the application of N-dimethylethylenediamine as cell lysosome fluorescent dye
[0037] 1) Add rhodamine 6G (2g) into a flask containing 20mL N,N-dimethylethylenediamine, stir the system electromagnetically, react at 80°C for 12h, remove unreacted solvent, separate and purify it through a silica gel column Rhodamine 6G-N,N-dimethylethylenediamine was obtained.
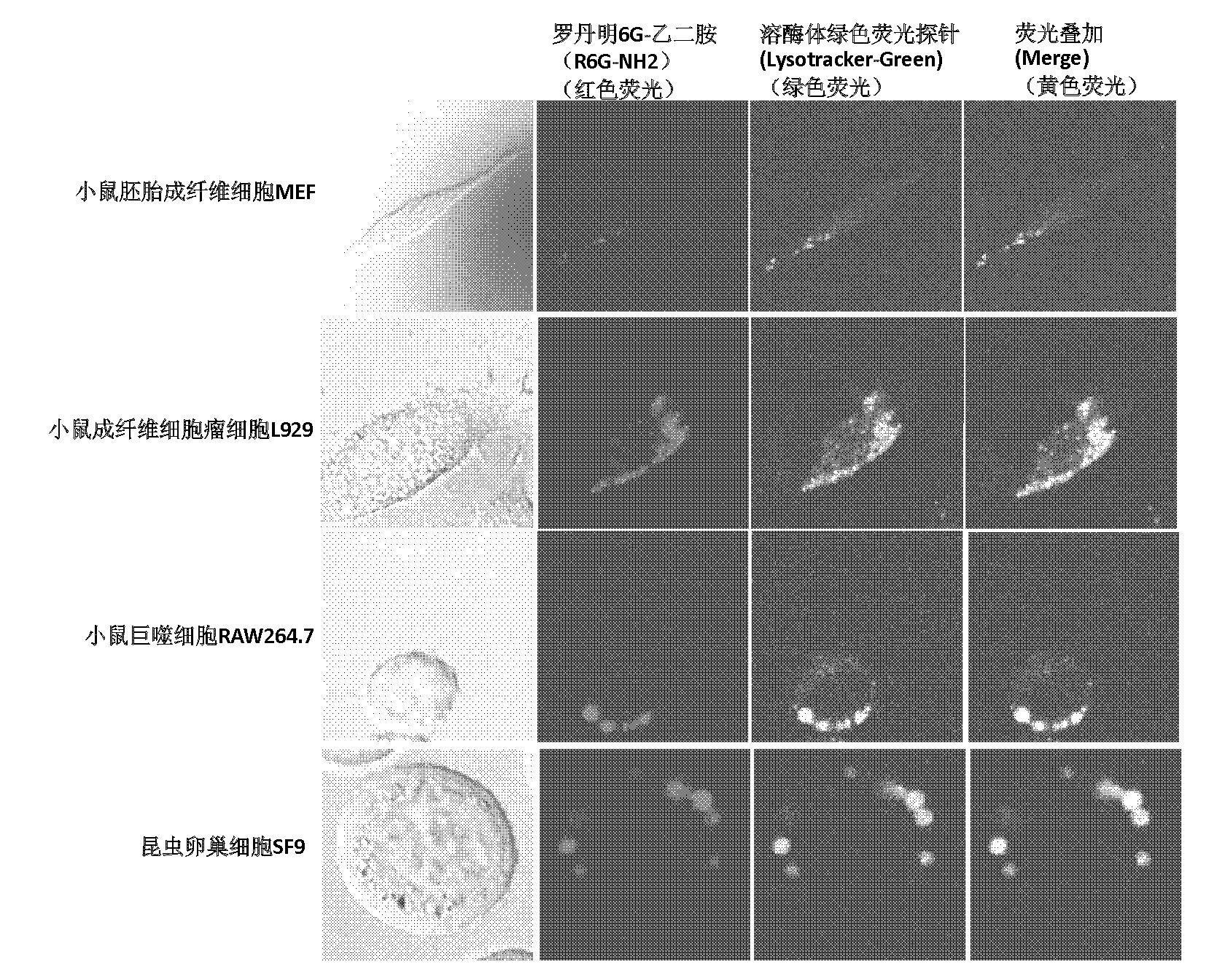
[0038] 2) Utilize rhodamine 6G-N, the steps 2) of N-dimethylethylenediamine repeat embodiment 1), 3), 4), 5), 6), 7), the result obtained is the same as that of rhodamine 6G-B The experimental results of the diamine derivatives are similar, indicating that the performance of the two as specific probes for lysosomes of cells is basically the same.
Embodiment 3
[0039] Example 3: Application of rhodamine 6G-propylenediamine as a fluorescent dye for cell lysosomes
[0040] 1) Add rhodamine 6G (2g) into a flask containing 20mL propylenediamine, stir it electromagnetically, react at 80°C for 12h, remove the unreacted solvent, separate and purify through a silica gel column to obtain rhodamine 6G-propylenediamine .
[0041] 2) Utilize rhodamine 6G-propylenediamine to repeat the steps 2) of embodiment 1), 3), 4), 5), 6), 7), the result obtained is similar to the experimental result of rhodamine 6G-ethylenediamine, It shows that the performance of the two as the specific probe of the lysosome of the cell is basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com