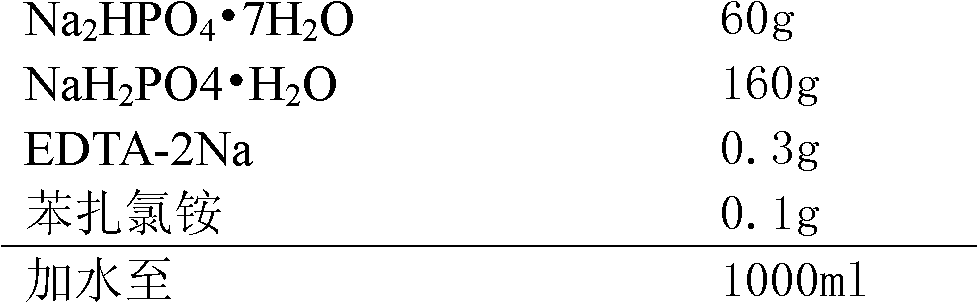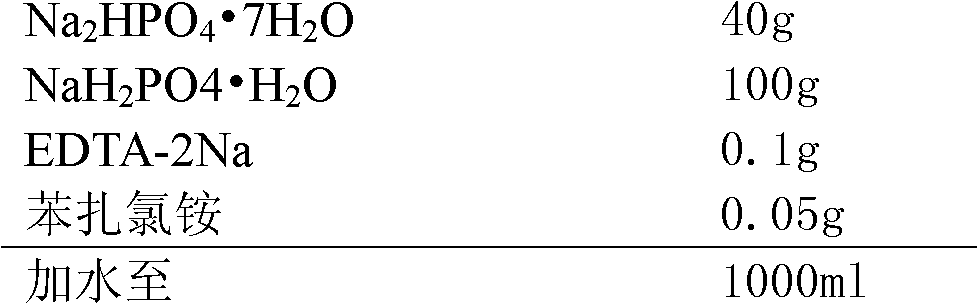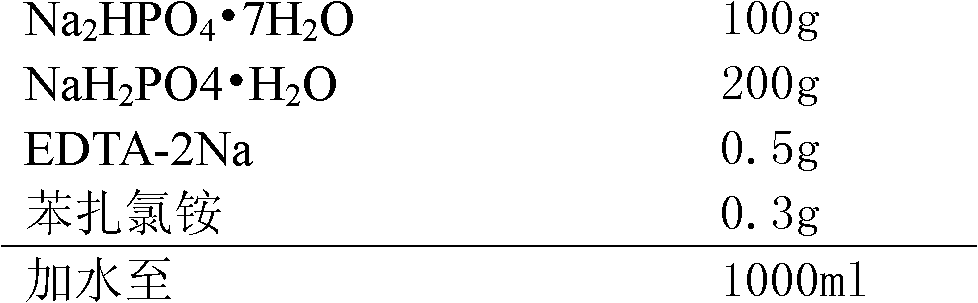Liquid preparation of sodium ascorbyl phosphate
A technology of liquid preparation and sodium phosphate salt, which is applied in the direction of active ingredients of phosphorus compounds, drug delivery, digestive system, etc., and can solve problems such as high equipment and energy consumption, and inconvenience in industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] prescription:
[0018]
[0019] Preparation Process:
[0020] (1) Dosing: First, stir and dissolve the prescribed amount of benzalkonium chloride and EDTA-2Na with 70% purified water, then add Na 2 HPO 4 ·7H 2 O and NaH 2 PO 4 ·H 2 O, stir to dissolve, add purified water to the full amount, stir and mix evenly to obtain the original solution before filtration, take a sample to detect the intermediate pH, relative density and content;
[0021] (2) Filtration: After the intermediate is qualified, filter the solution with a 0.45 μm microporous membrane;
[0022] (3) Filling and sealing: prepare plastic bottles and fill them. After filling, seal and cap in time, and send samples for inspection;
[0023] (4) Packing: After the samples are fully inspected after filling, the qualified products will be labeled and packed into cartons.
Embodiment 2
[0025] prescription:
[0026]
[0027] Preparation Process:
[0028] (1) Dosing: First, stir and dissolve the prescribed amount of benzalkonium chloride and EDTA-2Na with 70% purified water, then add Na 2 HPO 4 ·7H 2 O and NaH 2 PO 4 ·H 2 O, stir to dissolve, add purified water to the full amount, stir and mix evenly to obtain the original solution before filtration, take a sample to detect the intermediate pH, relative density and content;
[0029] (2) Filtration: After the intermediate is qualified, filter the solution with a 0.45 μm microporous membrane;
[0030] (3) Filling and sealing: prepare plastic bottles and fill them. After filling, seal and cap in time, and send samples for inspection;
[0031] (4) Packing: After the samples are fully inspected after filling, the qualified products will be labeled and packed into cartons.
Embodiment 3
[0033] prescription:
[0034]
[0035] Preparation Process:
[0036] (1) Dosing: First, stir and dissolve the prescribed amount of benzalkonium chloride and EDTA-2Na with 70% purified water, then add Na 2 HPO 4 ·7H 2 O and NaH 2 PO 4 ·H 2 O, stir to dissolve, add purified water to the full amount, stir and mix evenly to obtain the original solution before filtration, take a sample to detect the intermediate pH, relative density and content;
[0037] (2) Filtration: After the intermediate is qualified, filter the solution with a 0.45 μm microporous membrane;
[0038] (3) Filling and sealing: prepare plastic bottles and fill them. After filling, seal and cap in time, and send samples for inspection;
[0039] (4) Packing: After the samples are fully inspected after filling, the qualified products will be labeled and packed into cartons.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com