Convenient high-yield Arbekacin synthesis method
A technology of arbekacin and synthetic methods, applied in chemical instruments and methods, preparation of sugar derivatives, sugar derivatives, etc., can solve problems such as complex synthesis process and affecting product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
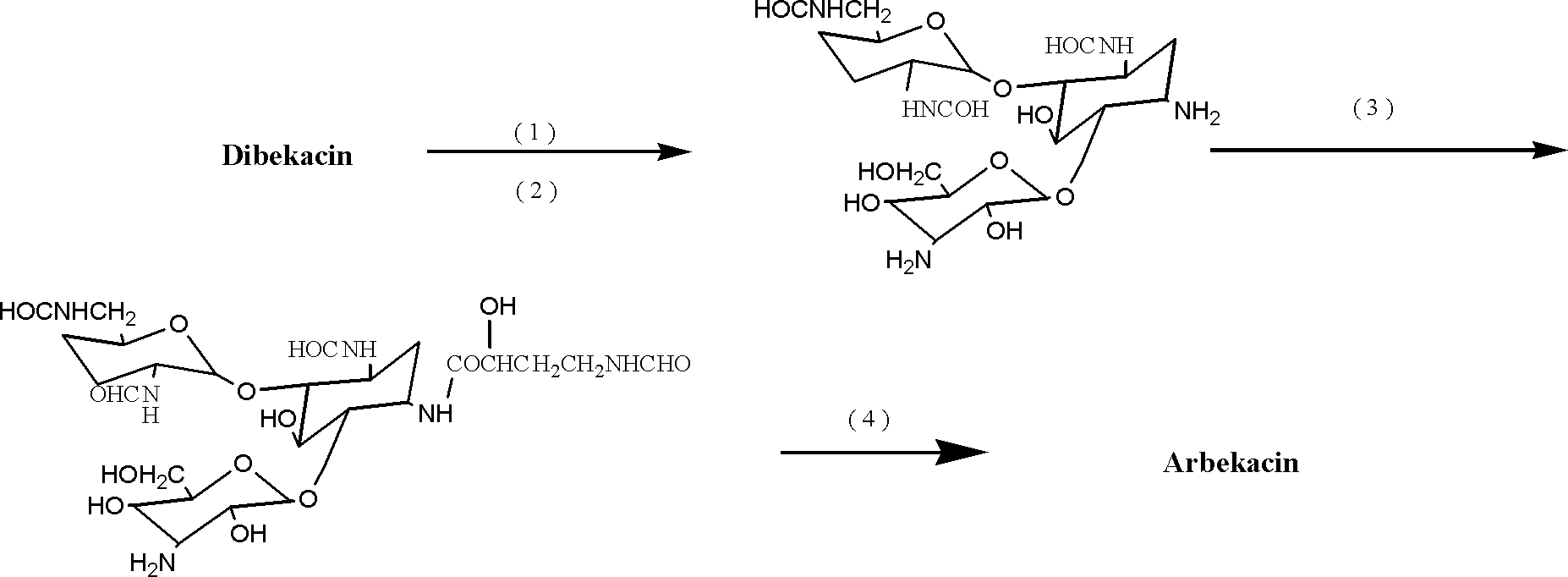
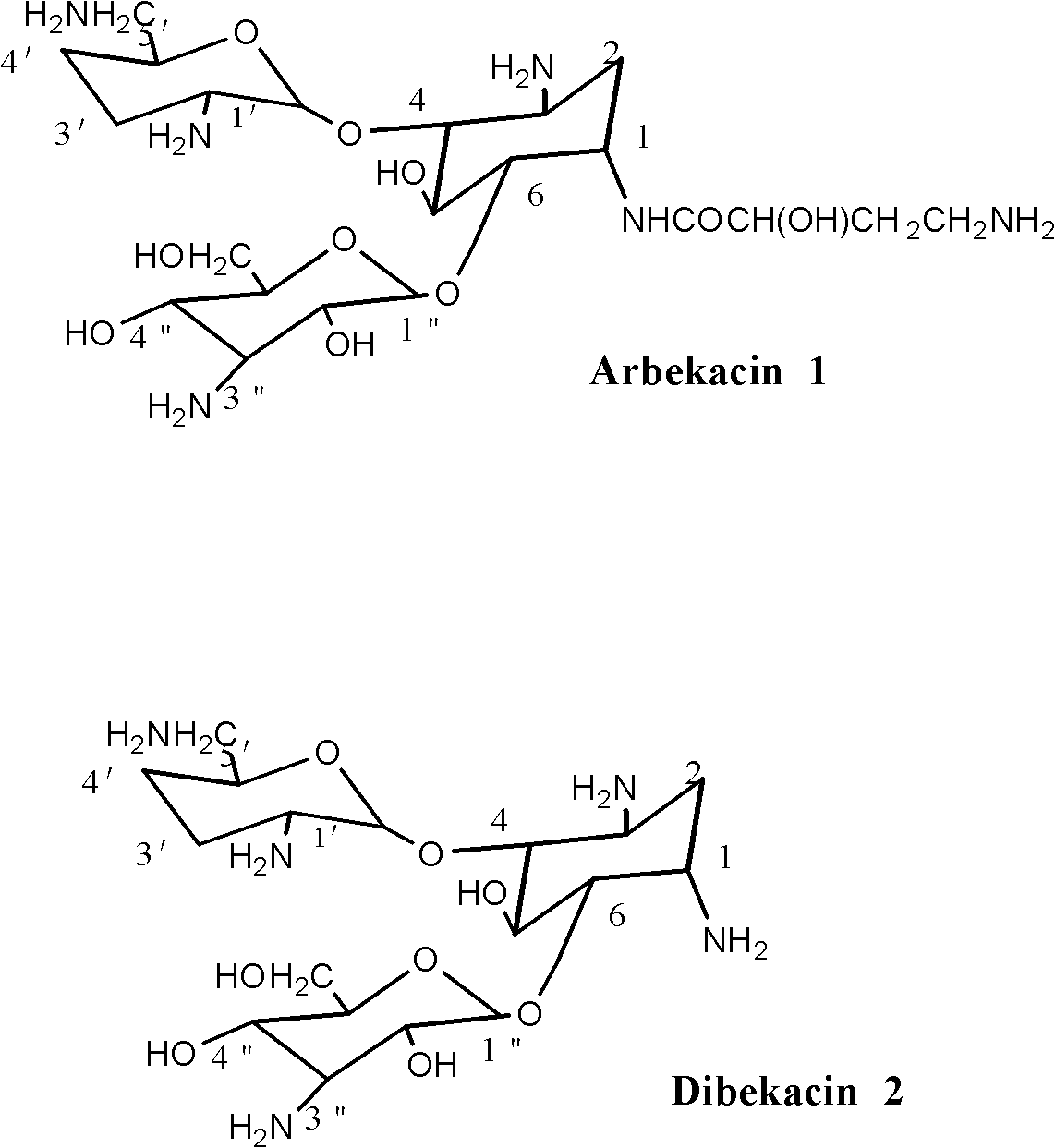
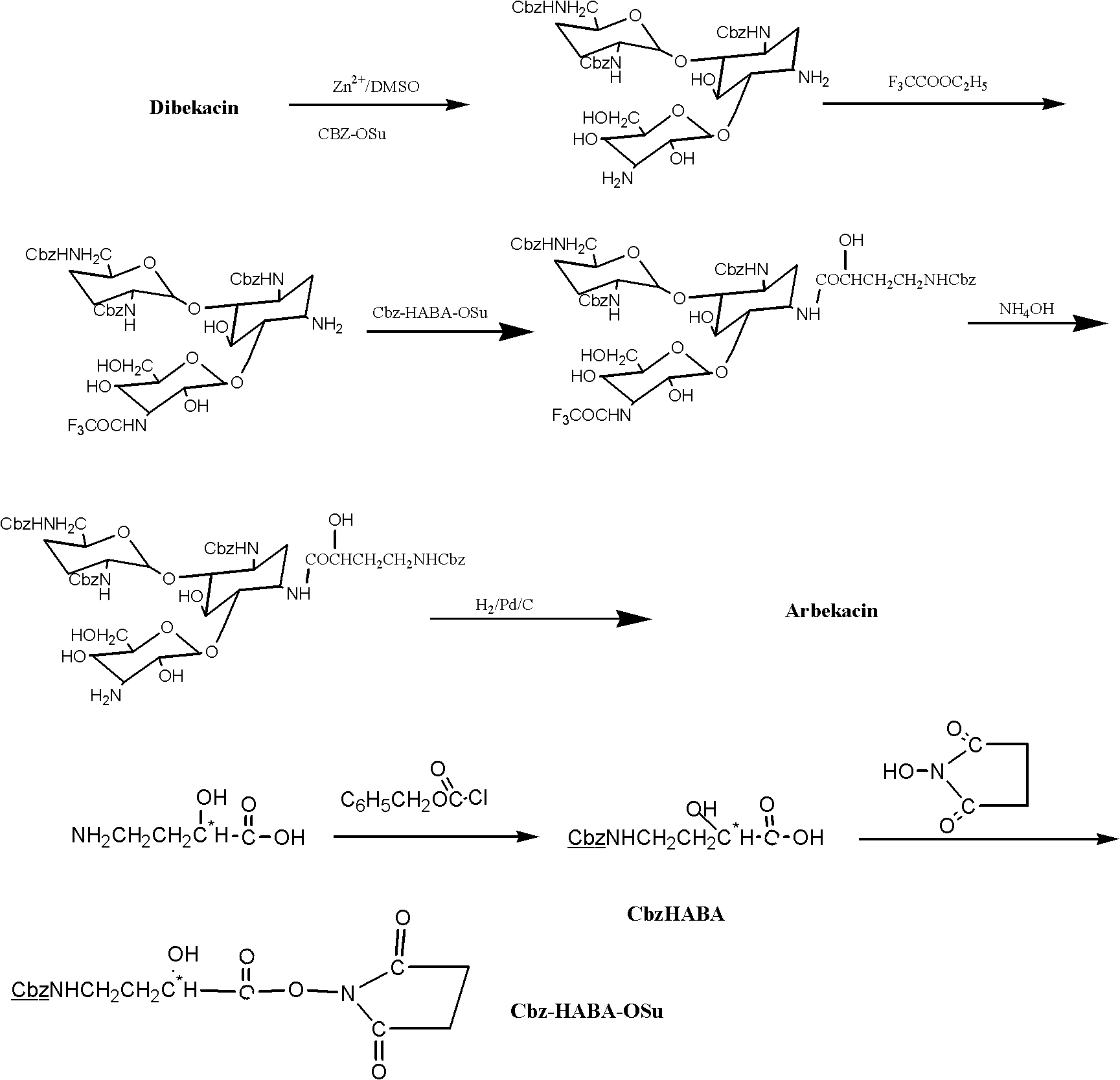
[0050] Example 1 Preparation of 3,2',6'-triformylamino-3',4'-dideoxykanamycin B
[0051] In a 500ml three-neck round bottom flask, add 3', 4'-dideoxykanamycin B (9.02 grams, 0.02mol), DMSO 150ml, stir for 10min, add 13.2g of zinc acetate, and stir at room temperature for 5 hours until The solution was clear. 2-formylmercaptobenzothiazole (12.88 g, 0.066 mol) was added, and the reaction was continued to stir at room temperature for 3 hours, and the end point of the reaction was judged by TLC.
[0052] The reaction solution was slowly poured into 400ml of water, a white precipitate was formed, stirred for about 0.5 hours, the pH was adjusted to 6-7 with ammonia water, the insoluble matter was filtered off, the filter residue was washed repeatedly with appropriate amount of water, and the filtrate was combined.
[0053] The filtrate is adsorbed on 800ml 732[NH 4 + ]-type resin, washed with a large amount of water and eluted with 0.3N ammonia water, collected the components wit...
Embodiment 2
[0055] The preparation of embodiment two (S)-4-formamido-2-hydroxybutyric acid
[0056] In a 250ml three-neck round bottom flask, add sodium carbonate (15.9 g, 0.15 mol) and 120 ml of water, and stir until completely dissolved. Add (S)-4-amino-2-hydroxybutyric acid (HABA) (11.9 g, 0.1 mol) to the above solution, stir and dissolve until completely clear, and check that the pH is above 10. Add 20ml of methanol solution and continue stirring for 30min. Slowly drop in 2-formylmercaptobenzothiazole (self-made, 21.5 g, 0.11 mol) dissolved in 10 ml of methanol solution, and the addition is completed in about 15 minutes. Stirring was continued at room temperature for an hour until the HABA spots disappeared completely in TLC. Developing agent: CHCl 3 -MeOH-NH 4 OH (25%) 4:4:1 (V / V / V), developer: 5% phosphomolybdic acid ethanol solution.
[0057] The above reaction solution was filtered to remove white solid, and the filtrate was concentrated to remove methanol. The residue was w...
Embodiment 3
[0058] The preparation of embodiment three Arbekacin
[0059] In a 250ml three-neck round bottom flask, add 3,2',6'-triformylamino-3',4'-dideoxykanamycin B (5.35 g, 0.01mol), methanol 40ml, 1-hydroxybenzo Triazole (HOBT 2.03 g, 0.015 mol), TEA 0.5 ml, stirred at room temperature until completely dissolved. Add (S)-4-formylamino-2-hydroxybutyric acid in tetrahydrofuran (12.94ml, 0.00085mol / ml), ice-water bath at 5-10°C, slowly add DCCI (3.12g, 0.015mol) ) was dissolved in 30ml of methanol solution, and the addition was completed in about 45 minutes. Stir the reaction at the same temperature for 6 hours, and check with TCL until the raw material spots completely disappear. Developing agent: CH 2 Cl 2 -MeOH-NH 4 OH (25%) 50:35:5 (V / V / V), iodine vapor color development.
[0060] The reaction solution was concentrated to dryness, and H 2 O 10ml, methanol 10ml, stirred at room temperature to dissolve. At 0-5°C, slowly add 50ml of 0.5M HCl dropwise, and control the addition f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com