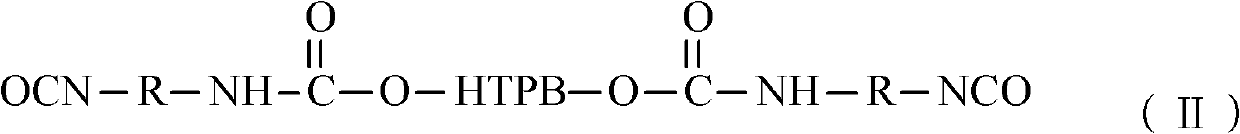Photocuring resin and preparation method thereof
A light-curing resin and diisocyanate technology, applied in the field of light-curing resin and its preparation, can solve the problems of poor surface drying and high curing energy, and achieve the effects of simple reaction process, low production cost and good surface drying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
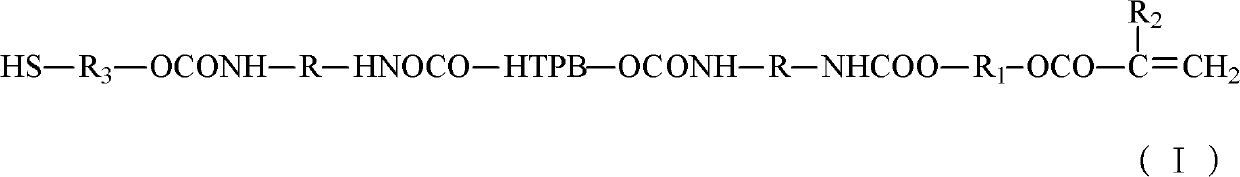
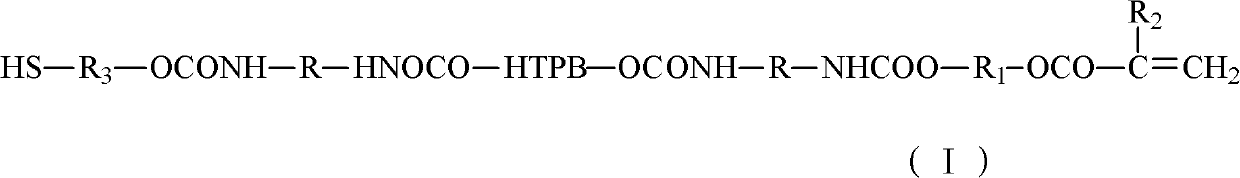
[0023] Vacuumize 100g of HTPB at 110°C (vacuum degree ≤ -0.08MPa) for 2 hours to remove the doped water; then cool down to 50°C, add 28.5g of TDI, and heat up to 85°C for 2 hours after the temperature is constant , until the NCO content reaches the design value (the design value is not the same, it is determined by the weight of the added HTPB and diisocyanate, the amount of the added substance is fixed, and the design value is fixed here) when the heating is stopped, that is, the two ends are NCO Group of polyurethane prepolymers:
[0024] After mixing 100g of polyurethane prepolymer with end-capping agent (a mixture of 4.2g of hydroxyethyl acrylate and 1.5g of mercaptoethanol), they were reacted at 70°C for 2 hours to obtain mercaptan-modified HTPB (hydroxyl-terminated polybutadiene ) type polyurethane acrylate.
Embodiment 2
[0026] Vacuumize 100g of HTPB at 100°C (vacuum degree ≤ -0.08MPa) for 3 hours to remove the doped water; then cool down to 60°C, add 24g of IPDI, and heat up to 80°C for 3 hours after the temperature is constant. Stop heating until the NCO content reaches the design value, and obtain a polyurethane prepolymer with NCO groups at both ends.
[0027] After mixing 100g of polyurethane prepolymer with end-capping agent (2g of hydroxyethyl methacrylate, 2.2g of hydroxyethyl acrylate and 2g of 6-mercaptohexan-1-ol), react at 60°C for 3 hours, A thiol-modified HTPB (hydroxyl-terminated polybutadiene) type polyurethane acrylate is obtained.
Embodiment 3
[0029] Vacuumize 100g of HTPB at 115°C (vacuum degree ≤ -0.08MPa) for 1.5 hours to remove the doped water; then cool down to 40°C, add 20g of MDI and 12g of IPDI, and heat up to 90°C for reaction after the temperature is constant After 1 hour, stop heating until the NCO content reaches the design value, and obtain a polyurethane prepolymer with NCO groups at both ends.
[0030] After mixing 100g polyurethane prepolymer with end-capping agent (the mixture of 4.3g hydroxypropyl acrylate and 3.1g 3-mercapto-1-hexanol), react at 80°C for 1 hour to obtain thiol-modified HTPB ( Hydroxyl-terminated polybutadiene) type polyurethane acrylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com