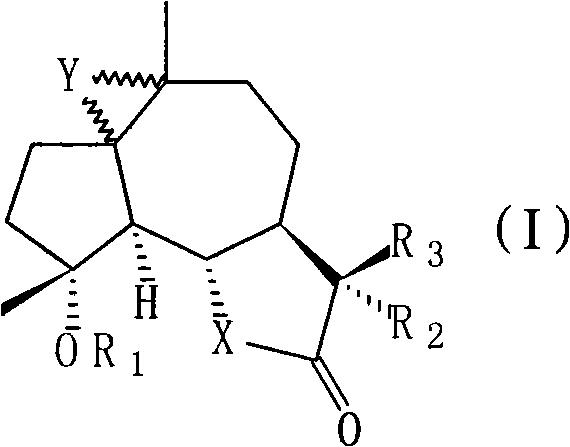
Sphaelactone derivatives, their pharmaceutical compositions, preparation method thereof and application thereof
A technology of derivatives and lactones, applied in the preparation of anti-cancer or auxiliary anti-cancer drugs, the field of pharmaceutical compositions for the treatment of cancer or adjuvant treatment of tumors, can solve the problems of drug resistance, insensitivity of tumor stem cells, etc., and achieve strong Inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
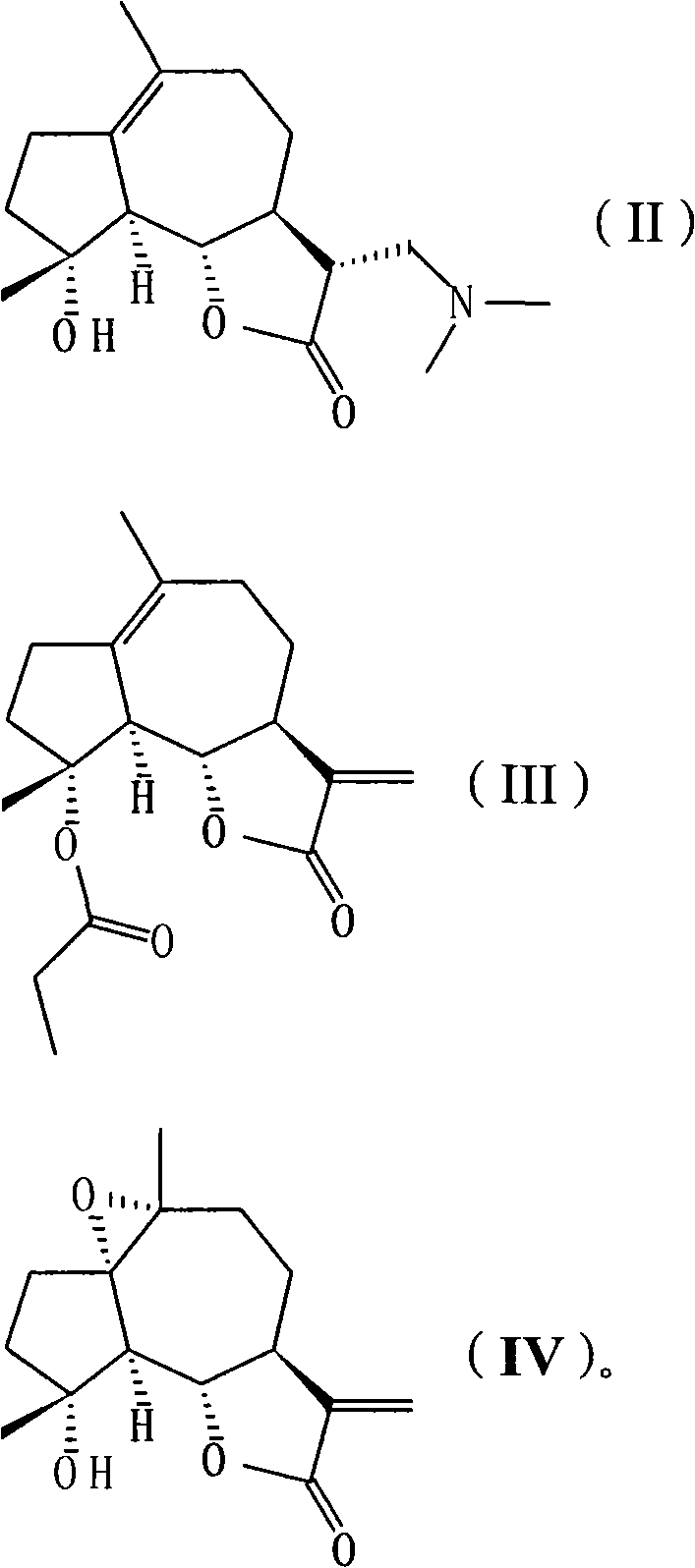
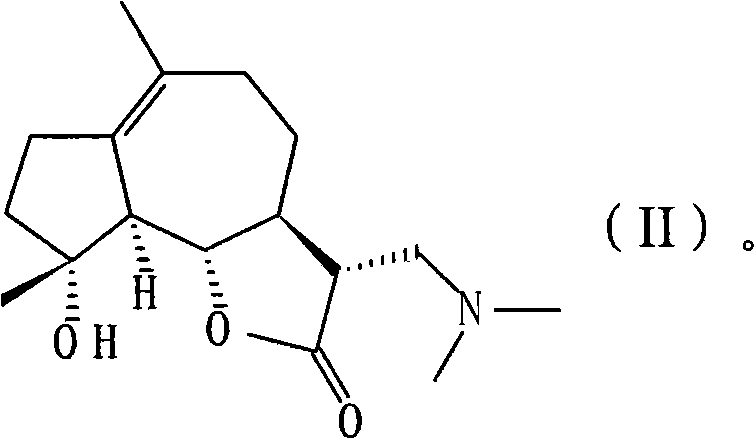
[0039] 11βH, the preparation of 13-dimethylaminomildolactone (compound 1, structural formula as following formula (II))
[0040]
[0041] Michelactone (106mg, 0.40mmol), triethylamine (2.0mL), and methanol (30mL) were added to a 100mL round-bottomed flask, heated to reflux for 3 hours, concentrated under reduced pressure, and silica gel column chromatography (petroleum ether: ethyl acetate Ester: triethylamine=50:50:0.5) to obtain 107.4 mg of white solid, yield: 86%.
[0042] Molecular formula: C 17 h 27 NO 3
[0043] Molecular weight: 293
[0044] Appearance: white amorphous powder
[0045] Spectral data:
[0046] 1 H NMR (CDCl 3 , 400MHz) δ3.76(t, J=10.0Hz, 1H), 2.96(s, 1H), 2.49-2.67(m, 3H), 2.28-2.34(m, 1H), 2.30-2.34(m, 2H) , 2.18(s, 6H), 2.09(brs, 2H), 1.96(d, J=11.2, 1H), 1.67-1.73(m, 2H), 1.60(s, 3H), 1.22(brs, 3H), 1.18 (br s, 2H); 13 C NMR (CDCl 3 , 100MHz) δ177.0, 131.8, 131.3, 84.0, 80.2, 58.3, 58.1, 50.9, 46.0, 44.6, 38.4, 35.3, 30.0, 27.2, 23.7, 22...
Embodiment 2
[0048] Preparation of 4-propionyl michelolactone (compound 2, structural formula as following formula (III))
[0049]
[0050] Michelolactone (106mg, 0.40mmol), triethylamine (2.0mL), propionyl chloride (0.2mL), and 5mL dichloromethane were added to a 20mL round-bottomed flask, stirred at room temperature for 24h, concentrated under reduced pressure, and silica gel column layer Analysis (petroleum ether: ethyl acetate = 90:10) gave 87 mg of white solid, yield: 72%. Structural data of the prepared 4-propionylmiglianolide:
[0051] Molecular formula: C 18 h 24 NO 4
[0052] Molecular weight: 304
[0053] Appearance: white amorphous powder
[0054] Spectral data:
[0055] 1 H NMR (CDCl 3 , 400MHz) δ6.14(s, 1H), 5.42(s, 1H), 3.74(t, J=10.0Hz, J=10.0Hz, 1H), 1.80-2.74(m, 12H), 1.67(s, 3H ), 1.50(s, 3H), 1.07(t, J=4.0Hz, 3H); 13 C NMR (CDCl 3 , 100MHz) δ173.8, 170.1, 139.5, 131.5, 130.4, 118.6, 88.4, 83.0, 56.6, 50.1, 36.5, 34.9, 30.4, 28.7, 25.9, 24.1, 18.8, 9.1.
Embodiment 3
[0057] 1, the synthetic method of 1,10-epoxy michelolactone (compound 3, structural formula following formula (IV)):
[0058]
[0059] Michelactone (106mg, 0.40mmol), m-chloroperoxybenzoic acid (0.45mmol), and 5mL of dichloromethane were added to a 20mL round-bottomed flask, stirred at room temperature for 6h, concentrated under reduced pressure, and silica gel column chromatography (petroleum ether : ethyl acetate=80:20), to obtain 96 mg of white solid, yield: 91%. Structural data of the prepared 1,10-epoxy michelolactone:
[0060] Molecular formula: C 15 h 20 NO 4
[0061] Molecular weight: 264
[0062] Appearance: white amorphous powder
[0063] Spectral data:
[0064] 1 H NMR (CDCl 3 , 400MHz) δ6.13(d, J=3.2Hz, 1H), 5.44(d, J=2.8Hz, 1H), 3.73(t, J=10.4Hz, 1H), 1.30-2.46(m, 11H), 1.29(s, 3H), 1.28(s, 3H); 13C NMR (CDCl3, 100MHz) δ168.7, 137.8, 118.6, 79.2, 77.3, 74.2, 66.7, 52.6, 48.4, 37.1, 33.8, 29.0, 24.6, 22.5 , 21.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com