Oncolytic adenoviral vectors and methods and uses related thereto
A viral vector and adenovirus technology, applied in the fields of life science and medicine, can solve the problem of insufficient induction of anti-tumor immunity, and achieve the effect of improving cancer treatment and reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
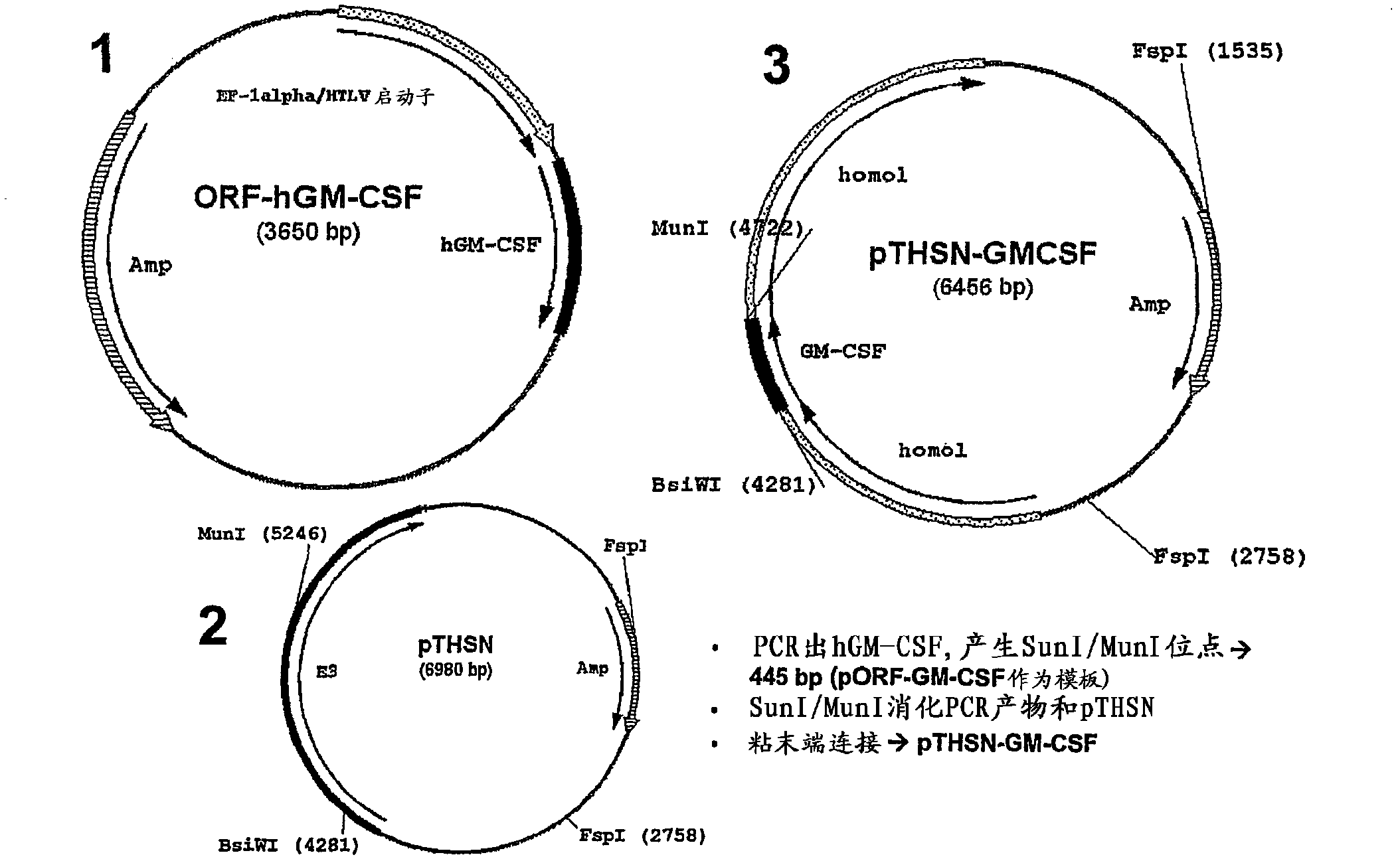
[0109] Embodiment 1. Cloning three kinds of D24-GM-CSF type viruses
[0110] -PCR out hGM-CSF,
[0111] - Generate SunI / MunI site => 445bp (pORF-GM-CSF as template)
[0112] -SunI / MunI digestion of PCR product and pTHSN
[0113] - sticky end ligation =>
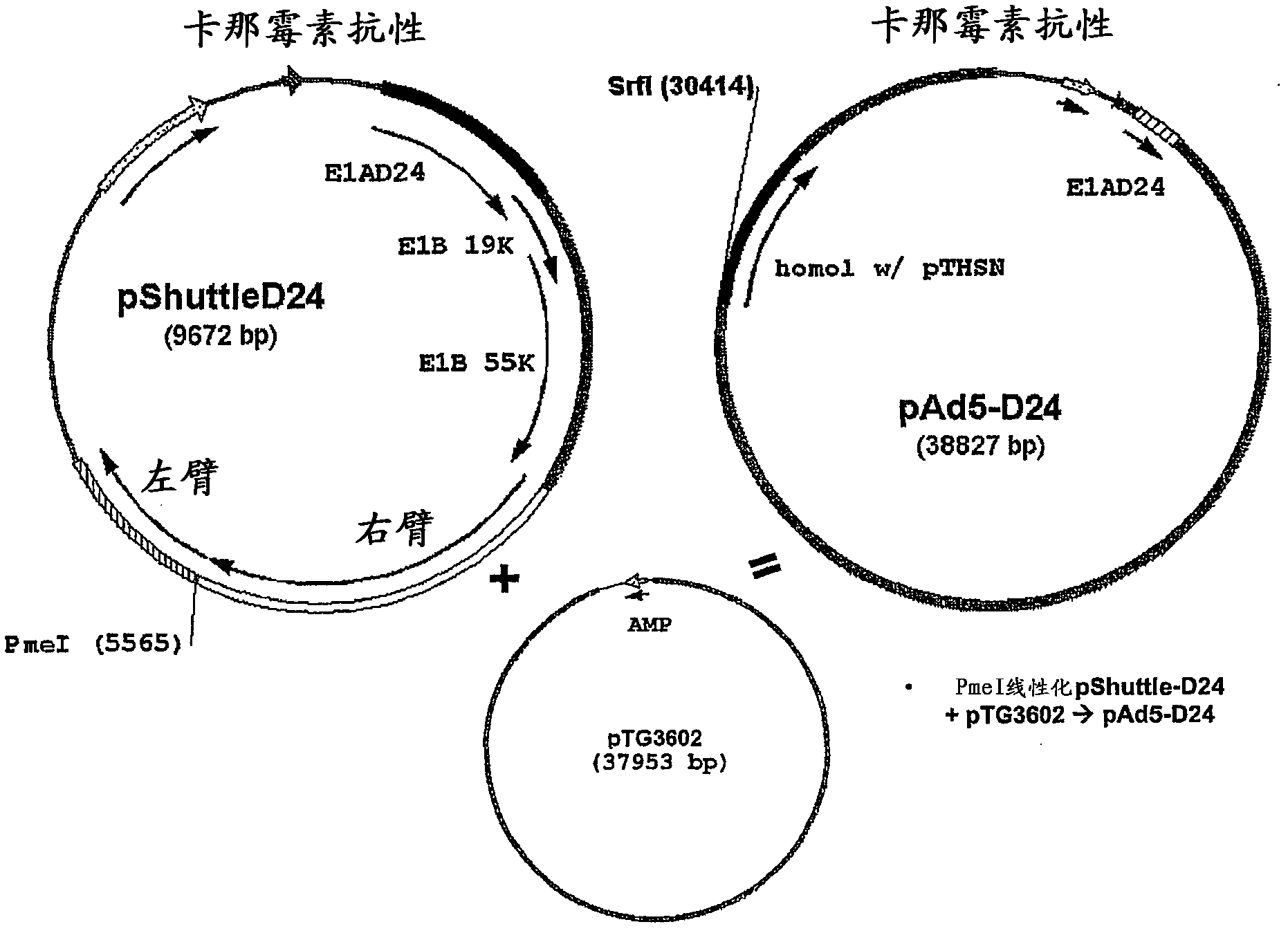
[0114] - PmeI linearized pShuttle-D24+pTG3602 => pAd5-D24
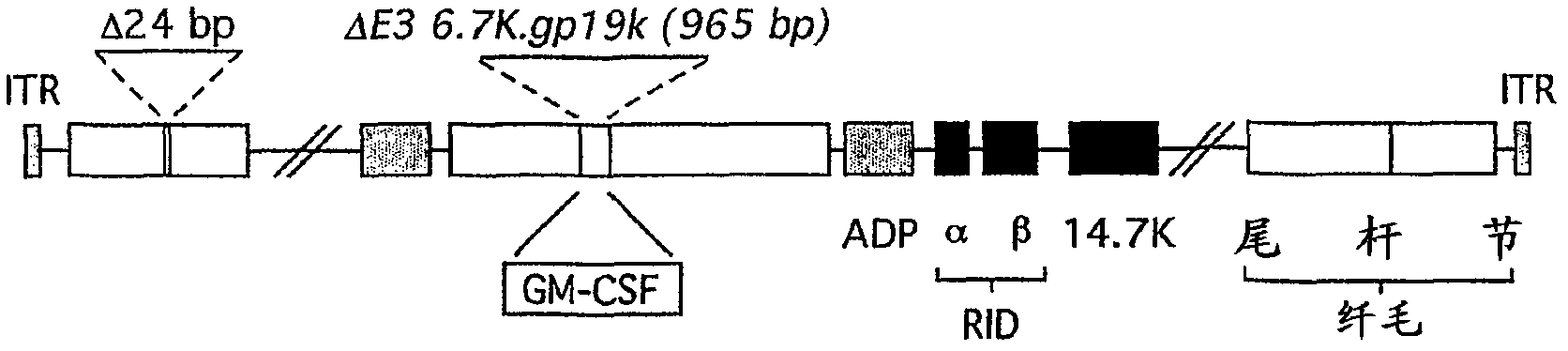
[0115] - Ad5-D24-GM-CSF (SEQ ID NO 8: E1A region with D24 deletion in nucleotide positions 563-1524 and ciliary region in nucleotide positions 30490-32236)
[0116] Homologous recombination: SrfI linearized pAd5-D24+FspI linearized pTHSN-GM-CSF=>pAd5-D24-GM-CSF
[0117] PacI linearization & transfection => Ad5-D24-GM-CSF
[0118] All stages of cloning were confirmed by PCR and multiple restriction digests. The shuttle plasmid pTHSN-GMCSF was sequenced. The absence of wild-type El was confirmed by PCR. The El region, transgene and cilia were checked by sequencing and PCR in the final virus, which was then taken to a clean laboratory for production. To this en...
Embodiment 2
[0121] Example 2. In vitro analysis of D24-GM-CSF virus
[0122] D24-GM-CSF-type virus was studied using the MTS cell-killing assay in lung cancer cells (A549), breast cancer stem cell-derived explants (J IMT-1), and breast cancer cells (MDA-MB-436) in vitro efficacy. The MTS assay is currently the standard method used to assess cell survival in cancer gene therapy publications. Ad5Luc1 is a replication defective virus and served as a negative control. Ad5wt is the wild type Ad5 virus (strain Ad300wt) and was used as a positive control. Ad5-d24-E3 contains an isogenic 24bp deletion in El but is complete in E3. VP denotes viral particle.
[0123] In conclusion, Ad5-D24-GMCSF had similar oncolytic activity to the positive control in vitro, and thus the production of the transgene did not compromise the oncolytic ability of the virus ( Figure 5a -c). Similar data were shown for Ad5 / 3-D24-GM-CSF and Ad5-RGD-D24-GM-CSF ( Figure 5d ).
[0124] To test whether Ad5D24-GMCSF ...
Embodiment 3
[0125] The pretreatment analysis of embodiment 3 transduction
[0126] I. Infection of tumor cells with Ad5Luc1
[0127] To check that tumors can be infected by Ad5-based viruses, biopsies taken from tissues were homogenized and infected with Ad5Luc1 encoding luciferase according to standard infection protocols. Briefly, the cells seeded in the wells were washed twice with PBS, the virus was thawed and resuspended in a minimal amount of growth medium and poured gently over the cells. Infection was carried out for 30 minutes, after which the cells were washed again in PBS and the appropriate amount of complete growth medium was added. Luciferase quantification was assessed after 24 hours. Note that only a small amount of tissue was obtained, so the number of cells could not be calculated, nor could the amount of virus corrected to the amount of tissue be calculated. Therefore, no quantitative analysis was performed, but qualitative data showed successful gene transfer in pat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com