Application of miRNA-20a and its inhibitors in the preparation of glioma stem cell invasion regulators
A technology of glioma stem cells, miRNA-20a, applied in the medical field to achieve the effect of high invasive properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
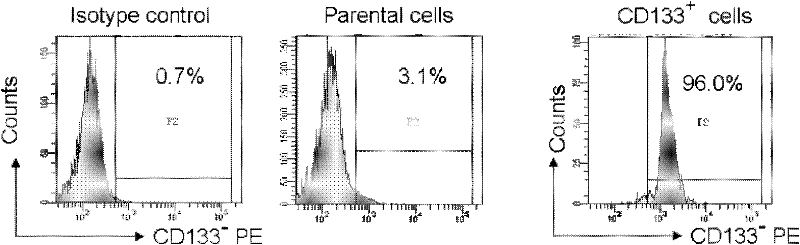
[0037] Example 1 Glioma Stem Cells
[0038] 1 material
[0039] Culture of primary human glioma cells
[0040] Fresh glioma tissues were obtained from the Department of Neurosurgery of Southwest Hospital and Daping Hospital (Chongqing Third Military Medical University). A total of 2 cases were taken successively, both of which were glioblastoma multiforme (WHO grade IV). The primary glioma tissue block was washed 3 times with 0.01M PBS, and the surface fibers and necrotic tissue were carefully removed; the tissue block was immersed in a small amount of DMEM medium (Gibco, USA), and the tumor tissue block was repeatedly cut to 1mm with ophthalmic scissors 3 fragments; add mixed collagenase (Gibco Company, USA) to digest for 20-30min; while digesting, blow with a pipette to obtain a single-cell suspension, and place it in DMEM medium (containing 10% fetal bovine serum by volume fraction) Cultivate; after 24 hours, change the medium to remove red blood cells, and use it to sort...
Embodiment 2
[0052] The establishment of embodiment 2 xenograft tumor animal model
[0053] 1 Source of experimental animals
[0054] Experimental Animals Female nude mice aged 4-6 weeks, weighing 15-20 g, were provided by the Experimental Animal Center of Third Military Medical University, and were bred under standard conditions.
[0055] 2 experimental groups
[0056] The experiment was divided into 3 groups, 5 in each group, the first group was U87-CD133 - cells and U87-CD133 + Cell group; the second group is primary Case1-CD133 - Cellular and primary Case1-CD133 + Cell group; the third group is: primary Case2-CD133 - cells and primary Case2-CD133 + cell group.
[0057] 3. Subcutaneous xenograft tumor model
[0058] The subcutaneous xenograft model was used to analyze the tumorigenic ability of GSCs. 5×10 4 CD133 + cells (derived from U87 cell line and primary glioma cells) and CD133 - The cells (derived from U87 cell line and primary glioma cells) were suspended in 100 μl o...
Embodiment 3
[0060] In vitro invasion of embodiment 3GSCs and GCs
[0061] To study the difference in the invasion ability of GSCs and GCs, we used Transwell in vitro invasion assay to observe the invasion ability of GSCs and GCs. The Transwell in vitro invasion test was divided into two groups, namely the GSCs group and the GCs group. The specific operation steps were as follows: the cells were discarded from the culture medium, and the cells were washed 3 times with PBS; the adherent cells were digested with trypsin + EDTA and then centrifuged. After the suspended cells were directly centrifuged, they were resuspended in the culture medium, and the cell concentration was adjusted to 5×10 5 / ml; unpack the transwell chamber (BD Company, USA), use sterile tweezers to clamp out the transwell chamber and put it into a 24-well plate; pipette 10 μl of Matrigel glue (BD Company, USA) mixture on the transwell membrane, pay attention to the injection Uniform, rapid and gentle action, Matrigel ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com