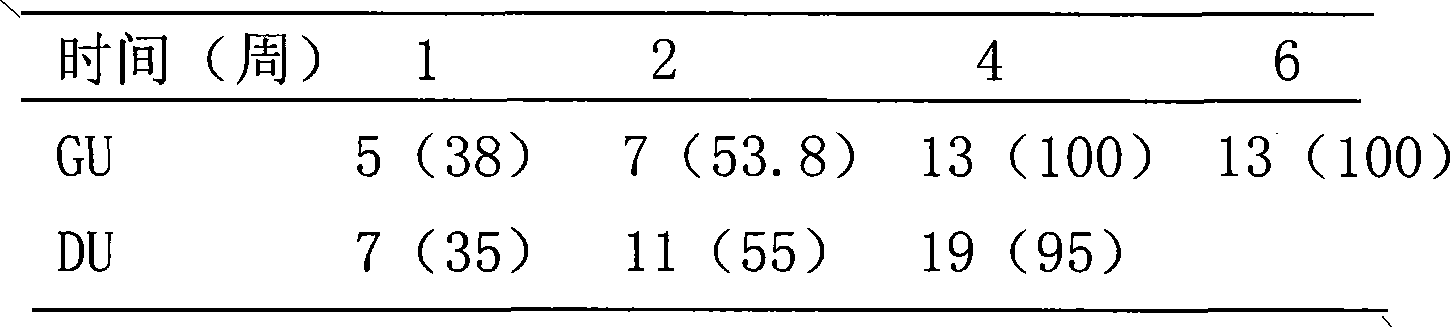Compound preparation of montmorillonite
A compound preparation and montmorillonite technology, applied in the field of medicine, can solve the problems of increasing production cost, complicated process and the like, and achieve the effects of orderly arrangement, uniform distribution and low direct toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
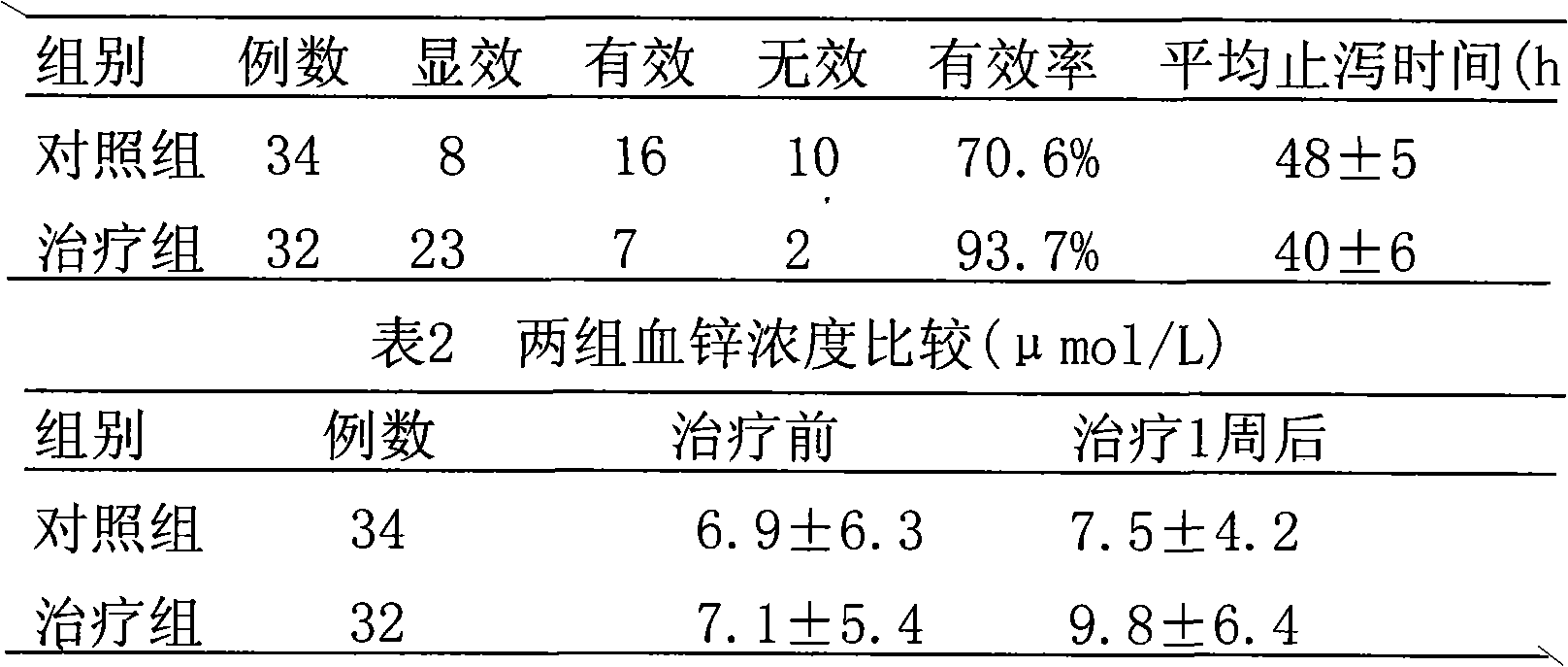
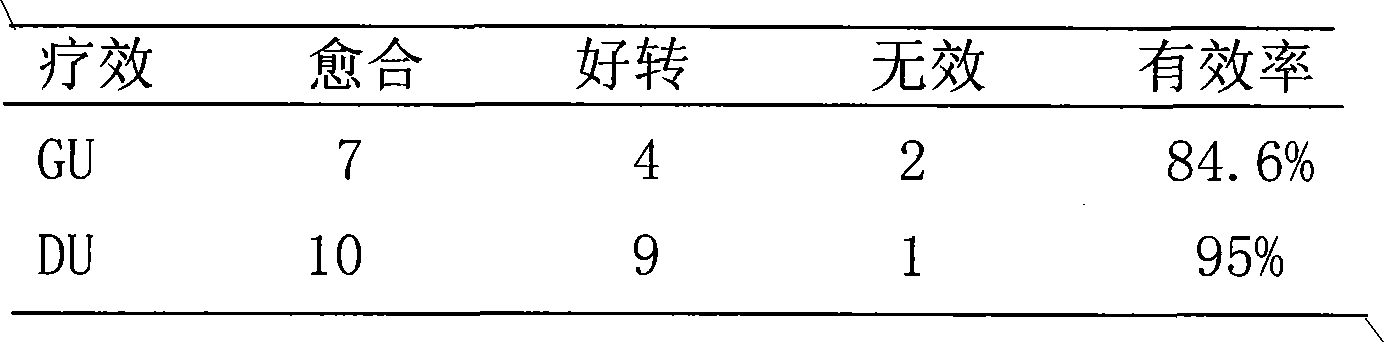
[0075] Take 1000g of montmorillonite, 94g of zinc citrate, and 100g of sucrose powder, and mix them thoroughly to obtain a powder. This compound medicine, montmorillonite: zinc=1: 0.03.
Embodiment 2
[0077] Take 1000g of montmorillonite and 200g of licorice zinc, mix well, wet granulate, dry, granulate, add an appropriate amount of lubricant, mix evenly, compress into tablets to obtain dispersible tablets. This compound medicine, montmorillonite: zinc=1: 0.01.
Embodiment 3
[0079] Take 1000g of montmorillonite, 33.5g of zinc chloride and 2g of sodium saccharin powder, and mix well to obtain a powder. This compound drug, montmorillonite: zinc = 1: 0.0016
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com