Human CTRP4 gene, its coding protein and their application
A protein and gene technology, applied in the fields of peptide/protein composition, application, gene therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: Cloning and identification of human new secreted protein CTRP4
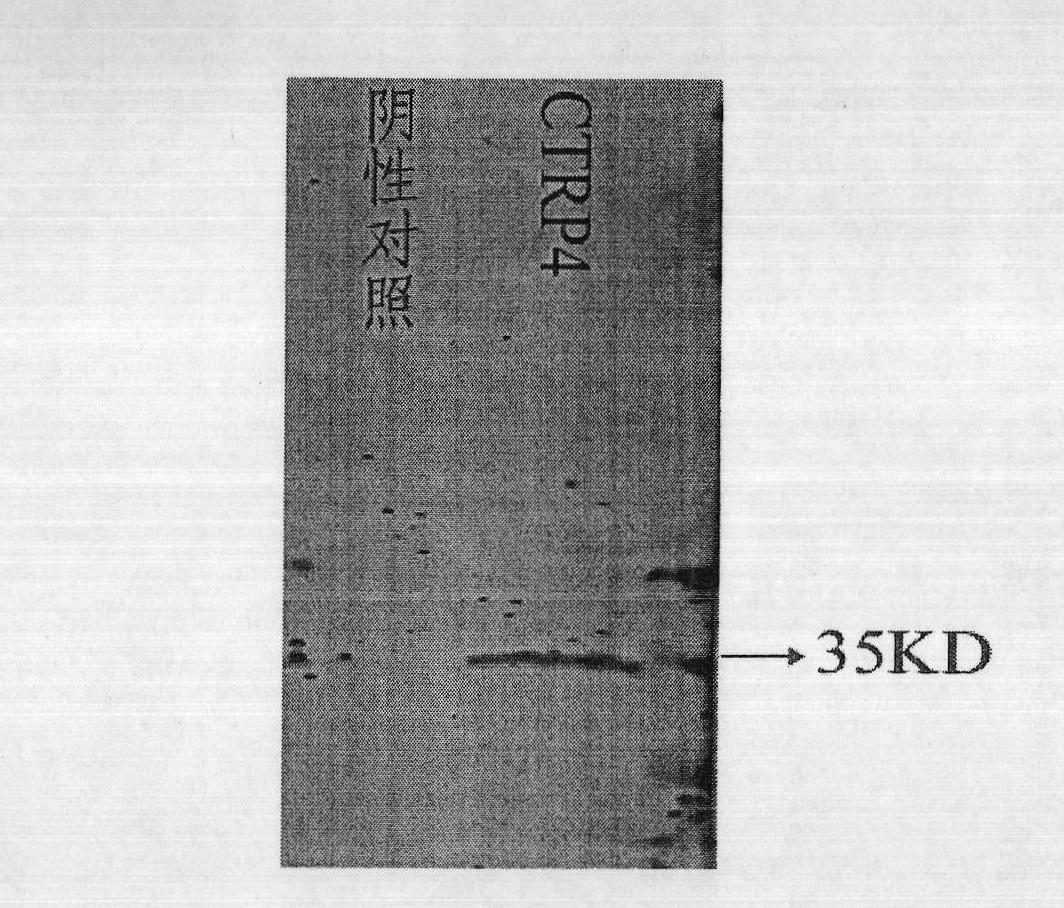
[0056] The inventors searched THMHMM and SignalP databases using bioinformatics methods, and found that the existing published CTRP4 protein does not have a typical transmembrane domain but contains a potential N-terminal signal peptide. This analysis strongly supports that the currently published CTRP4 may be a new potential secreted protein. In order to further confirm the currently published secreted form of CTRP4 and its signal peptide cleavage site, we performed N-terminal sequencing of CTRP4 secreted protein to verify the exact sequence of its secreted and mature CTRP4. The sequencing results clearly show that CTRP4 is a new secreted protein (SEQ ID NO.: 2), and its actual signal peptide cleavage site is located between the 16th and 17th amino acids of the existing published CTRP4 protein, which is consistent with biological information The predicted sites are consistent.
[0057] In ord...
Embodiment 2
[0058] Example 2: Overexpression of CTRP4 protein can effectively induce the activation of IL6-STAT3 and NF-KB signaling pathways
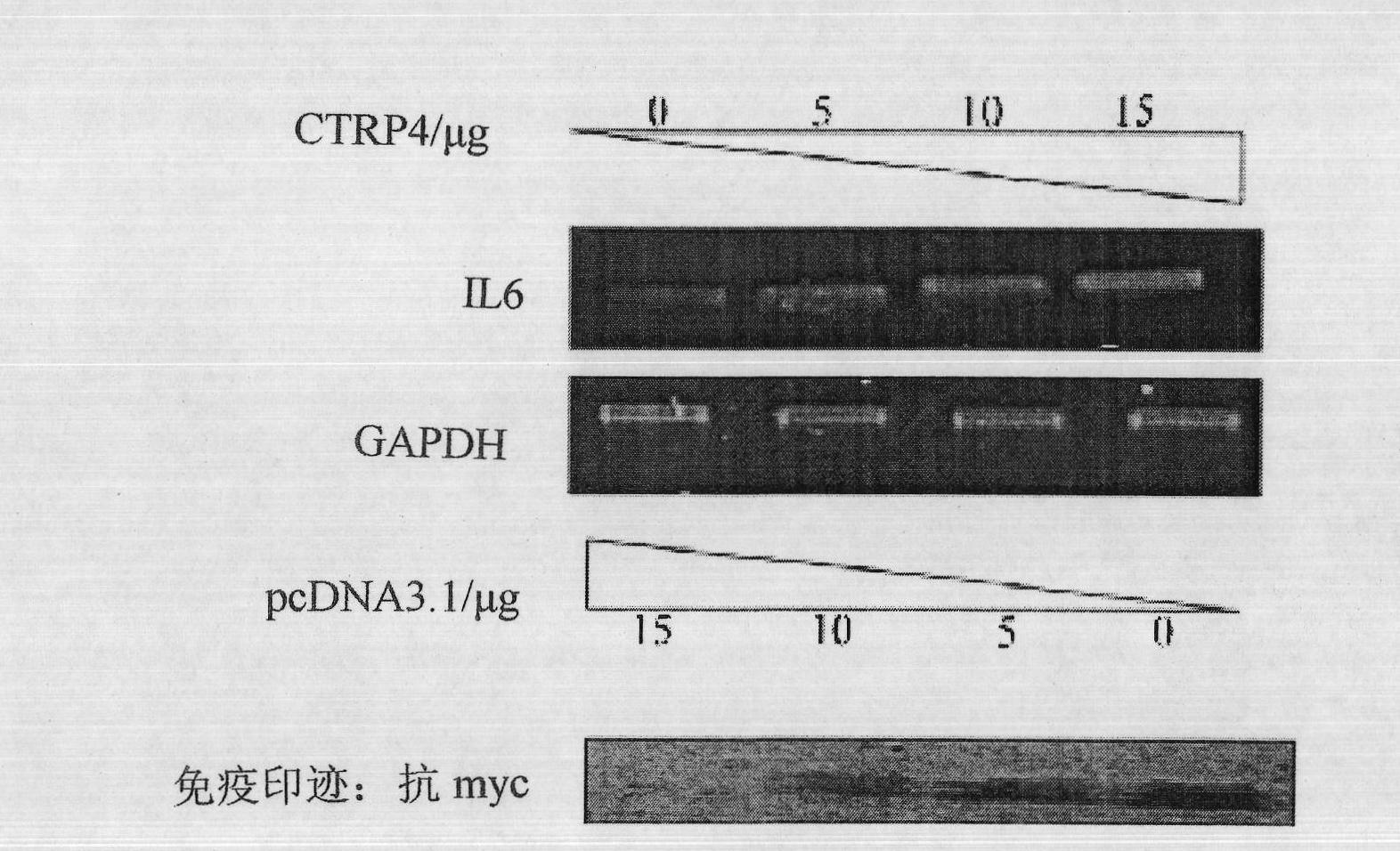
[0059] First of all, we studied the biological activity of CTRP4 gene and protein through RT-PCR test and PCR technique. As described in Example 1, the pcDNA3.1-CTRP4-myc fusion plasmid was overexpressed in human liver cancer cell HepG2 (commercially purchased), and the test results showed that in the HepG2 cell line overexpressing the CTRP4 gene, the mRNA level of IL6 was overexpressed Significant dose-dependent upregulation. ( figure 2 ).
[0060]Existing studies have shown that there is a very complex positive feedback loop between IL6-STAT3 and NF-κB interaction network, which plays a decisive role in the molecular regulation of tumorigenesis and tumor transformation. Given the close association of CTRP4 to the IL6-STAT3 signaling pathway, we further investigated the relationship between CTRP4 and NF-κB activation.
[0061] We detected th...
Embodiment 3
[0063] Therefore, the CTRP4 gene antagonist can be applied to the preparation of drugs for inhibiting the activity of NF-κB and / or IL6-STAT3 pathway. The antagonist is an siRNA molecule or an antisense RNA that blocks or blocks the mRNA transcribed by the CTRP4 gene. Techniques of how to block or block gene expression using antagonists are known in the art. Example 3: Biological activity of secreted protein CTRP4 (SEQ ID NO.2)
[0064] The above results show that CTRP4 is a new secreted protein, and overexpression of CTRP4 in some cancer cell lines can induce the activation of IL6-STAT3 pathway. Accordingly, we hypothesized that the secreted protein CTRP4 may be involved in the regulation of tumor-associated inflammation as a potential secreted regulatory factor.
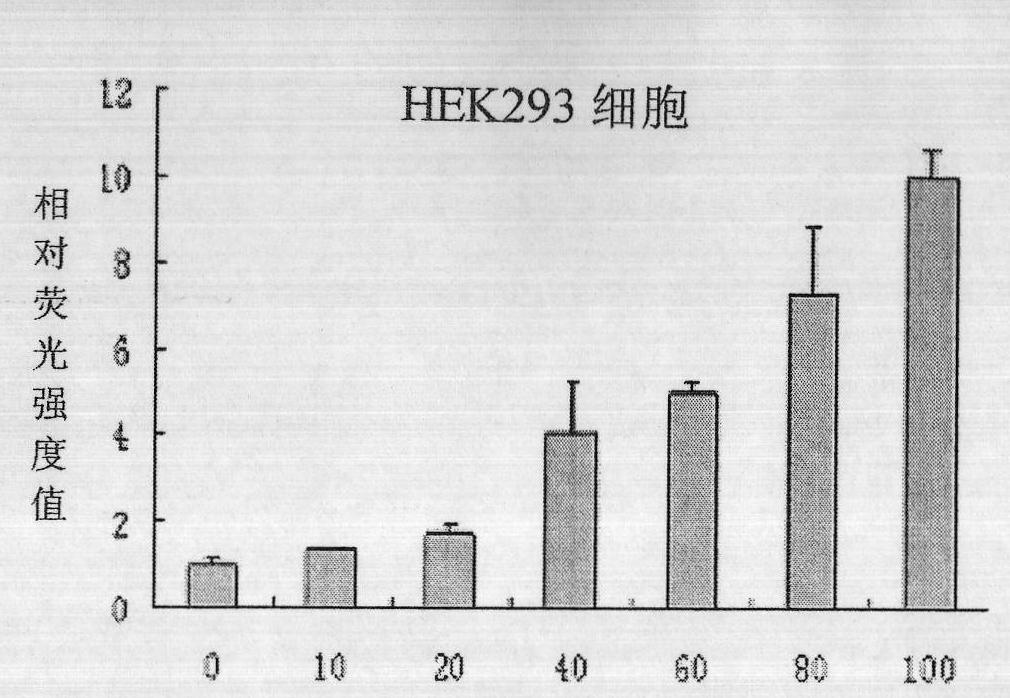
[0065] First, we tested whether the culture supernatant of HepG2 cells overexpressing CTRP4 had similar biological functions as we expected to overexpress CTRP4. We transferred the overexpression supernatant to H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com