Method for separating U (uranium) from Pu (plutonium) in Purex process
A uranium and plutonium process technology, applied in the field of uranium and plutonium separation in the Purex process, can solve problems such as unfavorable process, slow reduction rate, and demand exceeding stoichiometry, and achieve the effect of simplifying the process and improving separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] Table 1 Reduction stripping effect when different phases are compared
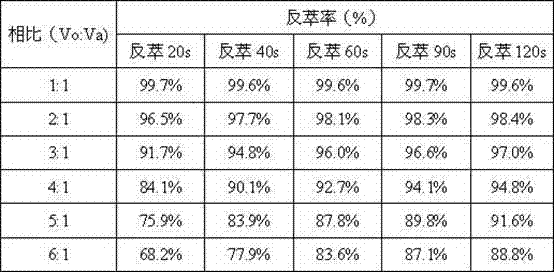
[0024] When the concentration of Pu in the organic phase solution is c (Pu(IV)) 0 =0.80g / L, the concentration of HSC in the aqueous phase solution is c (HSC)=0.050mol / L, the hydrogen ion concentration is c (H + ) = 0.40mol / L, and the temperature is t = 21°C, the volume ratio of the organic phase solution to the aqueous phase solution (that is, the comparison) Vo:Va = 1:1~6:1 The experimental data of the reduction and stripping effect. When the ratio of the organic phase to the aqueous phase is less than or equal to 4:1, the stripping effect is better.
Embodiment 2
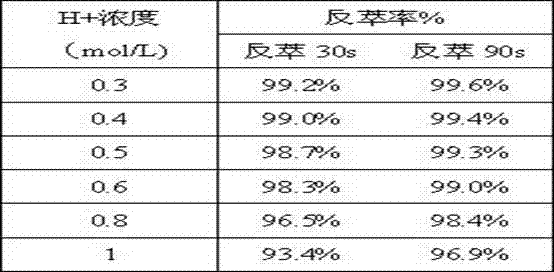
[0026] when c (Pu(IV)) 0 =0.80g / L, c (HSC)=0.050mol / L, compared to (Vo:Va)=1:1, t=21℃, different H + The experimental data of the reduction stripping effect during concentration is as shown in table 2. With H + As the concentration increases, the effect of reduction and stripping becomes worse.
[0027]
[0028] Table 2 Different H + Reduction stripping effect at concentration
Embodiment 3
[0030] when c (Pu(IV)) 0 =0.80g / L, c (H + ) =0.40mol / L, compared to (Vo:Va)=1:1, t=21°C, the reduction stripping effect when the ratio of the concentration of HSC in the aqueous phase solution to the concentration of Pu in the organic phase is 5~60 As shown in table 2.
[0031]
[0032] Table 3 Reduction stripping effect at different HSC concentrations
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com