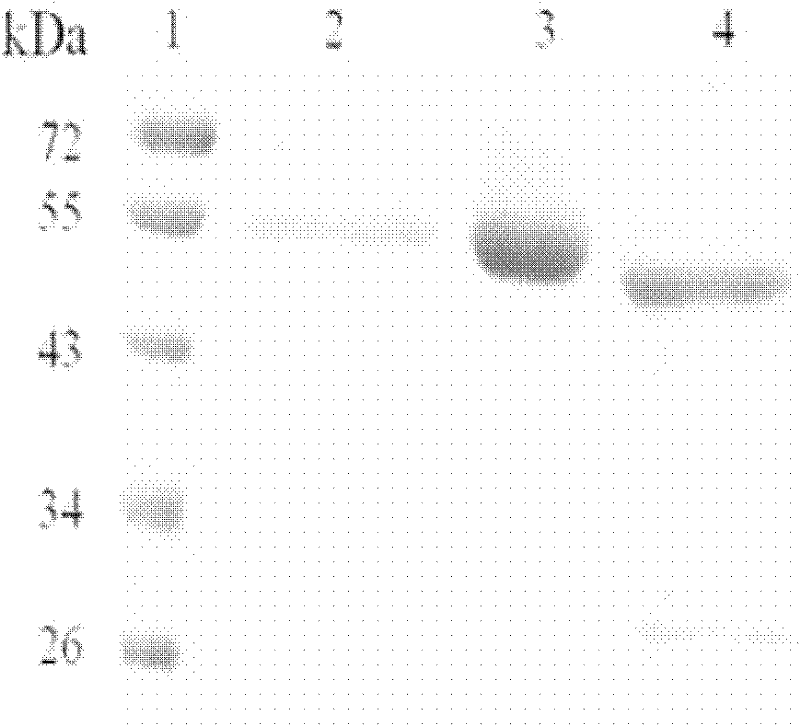
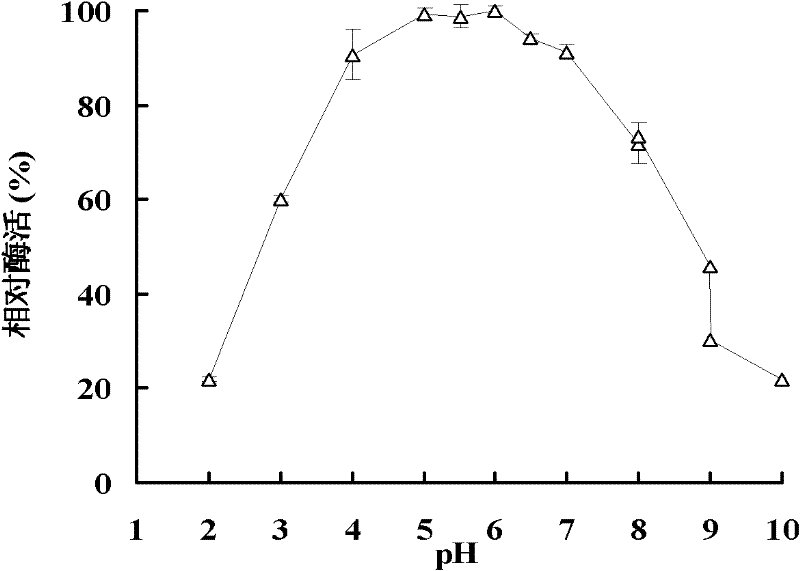
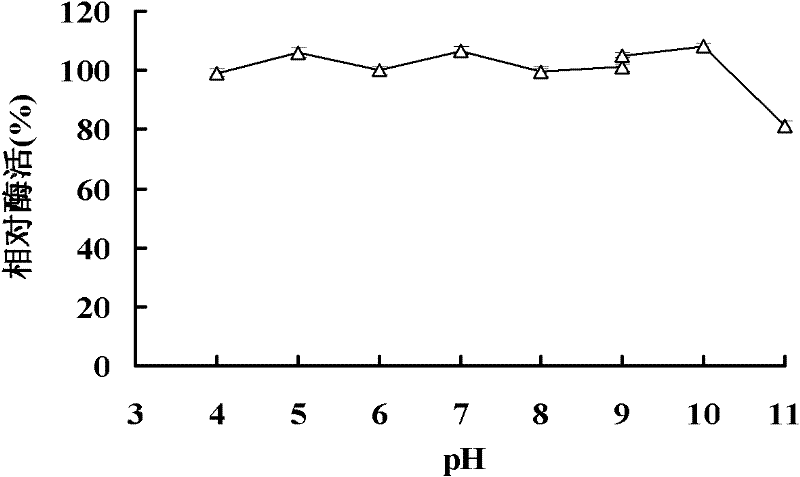
Beta-mannaseBA-Man5A with wide pH range, gene thereof and application of gene
A technology of mannanase and ppic9-ba-man5a, which is applied in the field of genetic engineering and can solve the problems of low yield of mannanase and inability to meet industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Example 1 Enzyme-producing properties of the fungus Bispora antennata CBS 126.38
[0127] After Bispora antennataCBS 126.38 was cultured in malt extract medium, it was inoculated in induction medium (0.2% MgSO 4 ·7H 2 O, 0.1% KH 2 PO 4 , 0.1% CuSO 4 ·5H 2 O, 0.1% CaCl 2 , 0.5% peptone, 1% corn cob flour, 1% bran, 0.5% konjac flour, pH 5.0), cultivated at 30°C for 3-4 days, and measured its cellulose and hemicellulase activity. It was determined that the bacteria was a high-production mannanase strain.
Embodiment 2
[0128] Example 2 Cloning of the gene ba-man5A encoding the fungus Bispora antennata CBS 126.38β-mannanase
[0129] Filter the mycelium cultured in liquid for 3 days with sterile filter paper, put it into a mortar, add 2mL of extract, grind for 5min, then put the grinding solution in a 50mL centrifuge tube, lyse in a water bath at 65°C for 20min, and mix every 10min. Homogenize once and centrifuge at 10,000 rpm for 5 min at 4°C. The supernatant was extracted in phenol / chloroform to remove impurity proteins, and then an equal volume of isopropanol was added to the supernatant. After standing at room temperature for 5 minutes, centrifuge at 10,000 rpm for 10 minutes at 4°C. The supernatant was discarded, the precipitate was washed twice with 70% ethanol, dried in vacuum, dissolved by adding an appropriate amount of TE, and stored at -20°C for later use.
[0130] The degenerate primers P1 and P2 were designed and synthesized according to the published conserved sequence of mannan...
Embodiment 3
[0135] RT-PCR analysis of embodiment 3 β-mannanase gene
[0136] Extract the total RNA of Bispora antennata CBS 126.38, use reverse transcriptase to obtain a strand of cDNA, then design appropriate primers M5AF and M5AR (Table 1) to amplify the single-stranded cDNA, obtain the cDNA sequence of mannanase, and amplify After the product was recovered, it was sent to Sanbo Biotechnology Co., Ltd. for sequencing.
[0137] After comparing the genome sequence and cDNA sequence of mannanase enzyme, it was found that the gene has 5 introns, the cDNA is 1299bp long (SEQ ID NO.4), encodes 432 amino acids (SEQ ID NO.1) and a stop codon The 18 amino acids at the N-terminal are its predicted signal peptide sequence, and the nucleotide sequence of the mature protein part of the measured gene ba-man5A is homologously compared with the mannanase gene sequence on GeneBank, which is derived from The putative protein of Botryotinia fuckeliana has the highest sequence identity of 60%, and the hig...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap