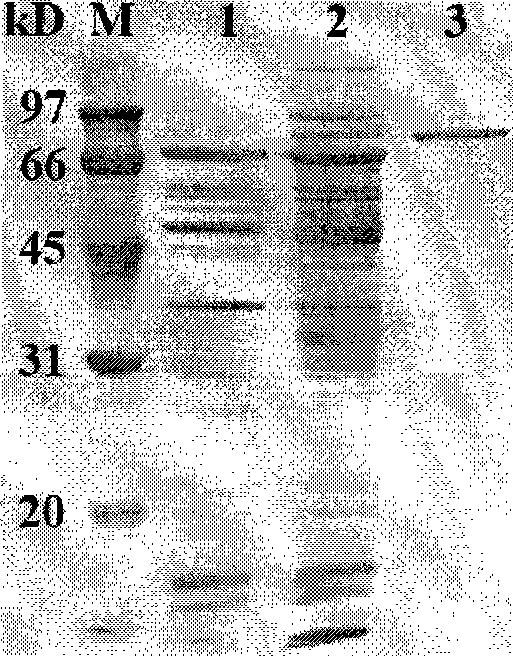
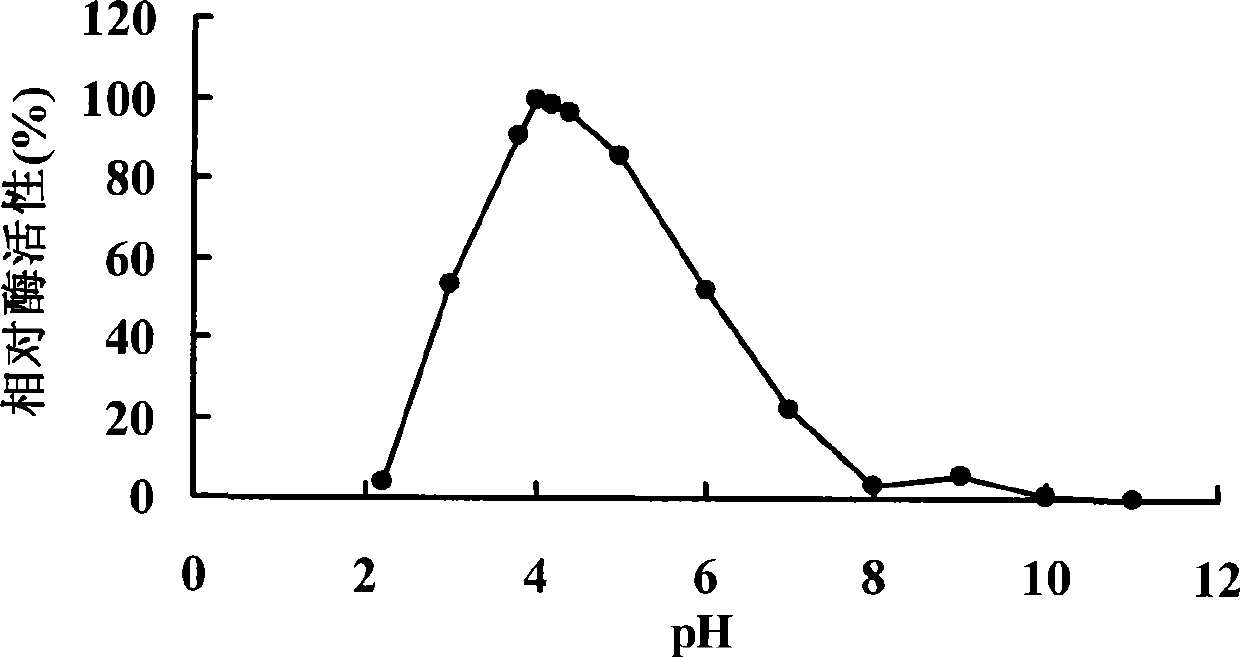
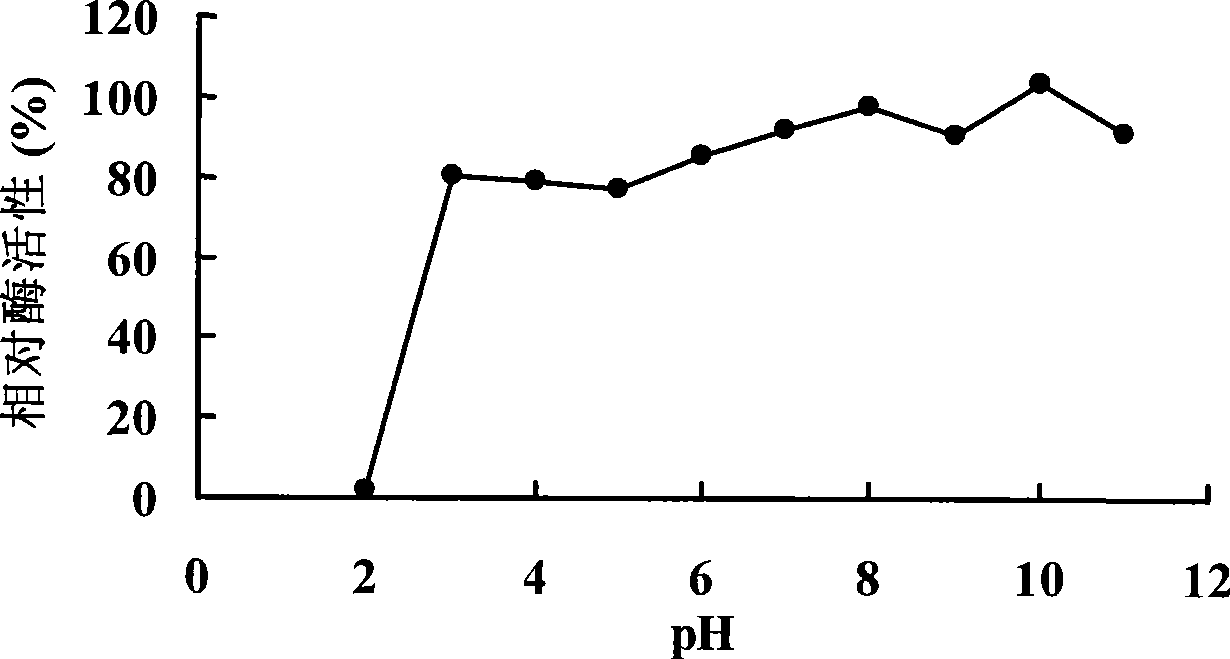
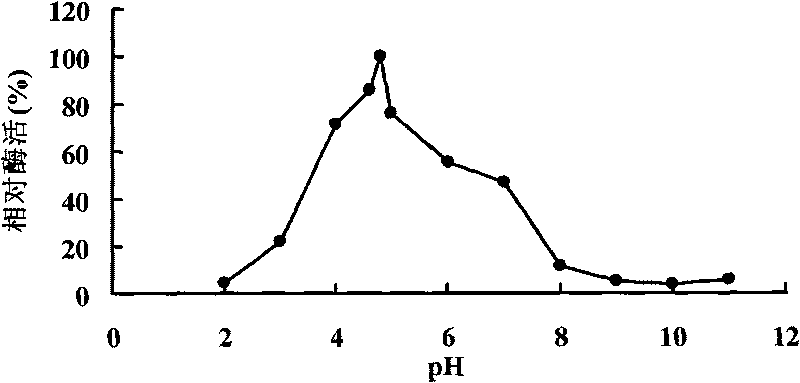
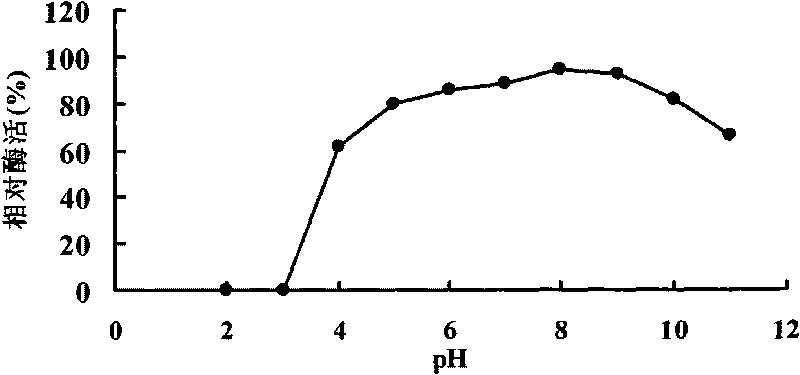
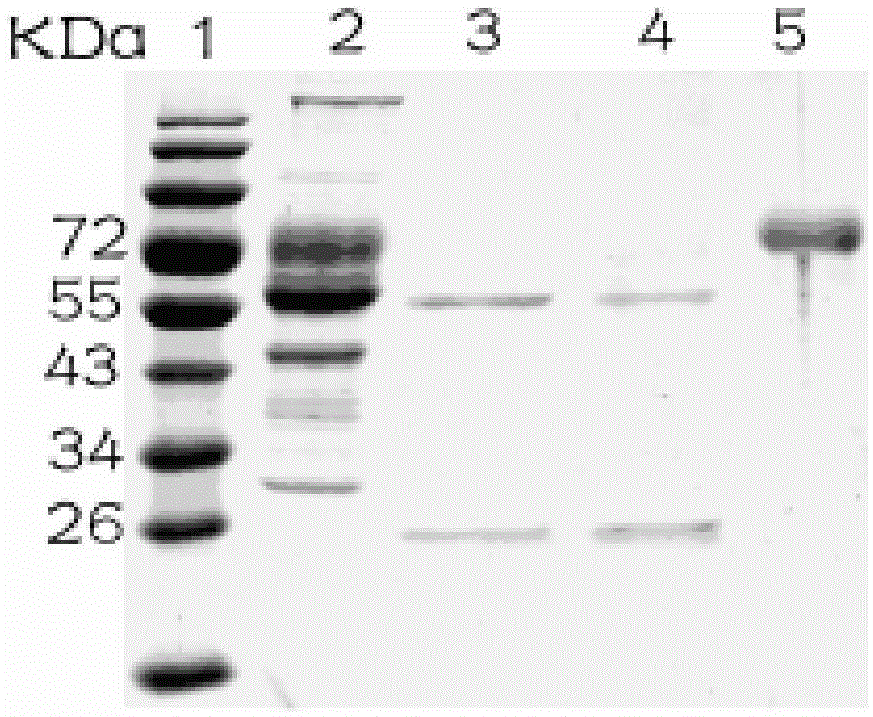
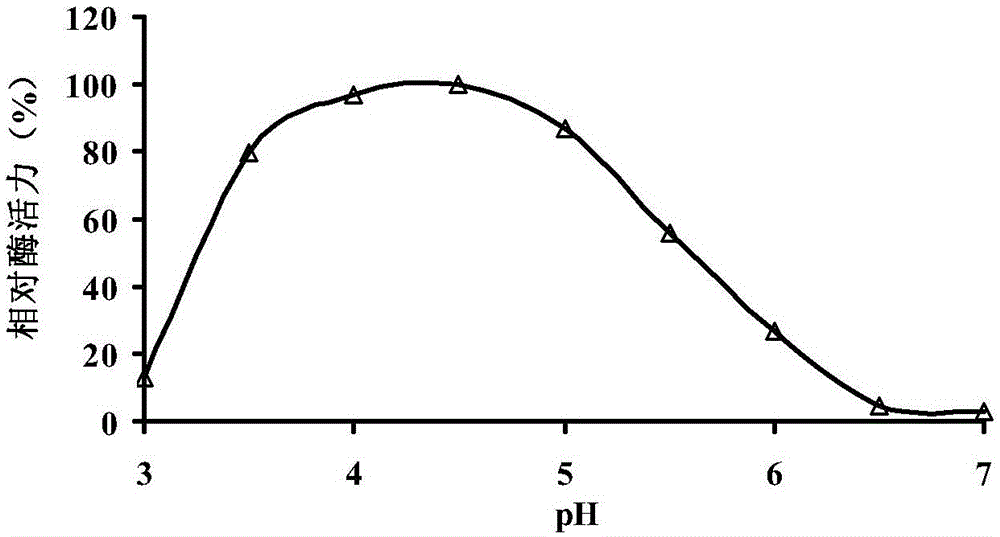
Patents
Literature
63 results about "Anti proteases" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Medical Definition of antiprotease. : a substance that inhibits the enzymatic activity of a protease.
Superoxide dismutase and preparation method thereof
InactiveCN101633916ARealize the industrialization of productionGood pH stabilityFungiBacteriaDismutaseNucleotide
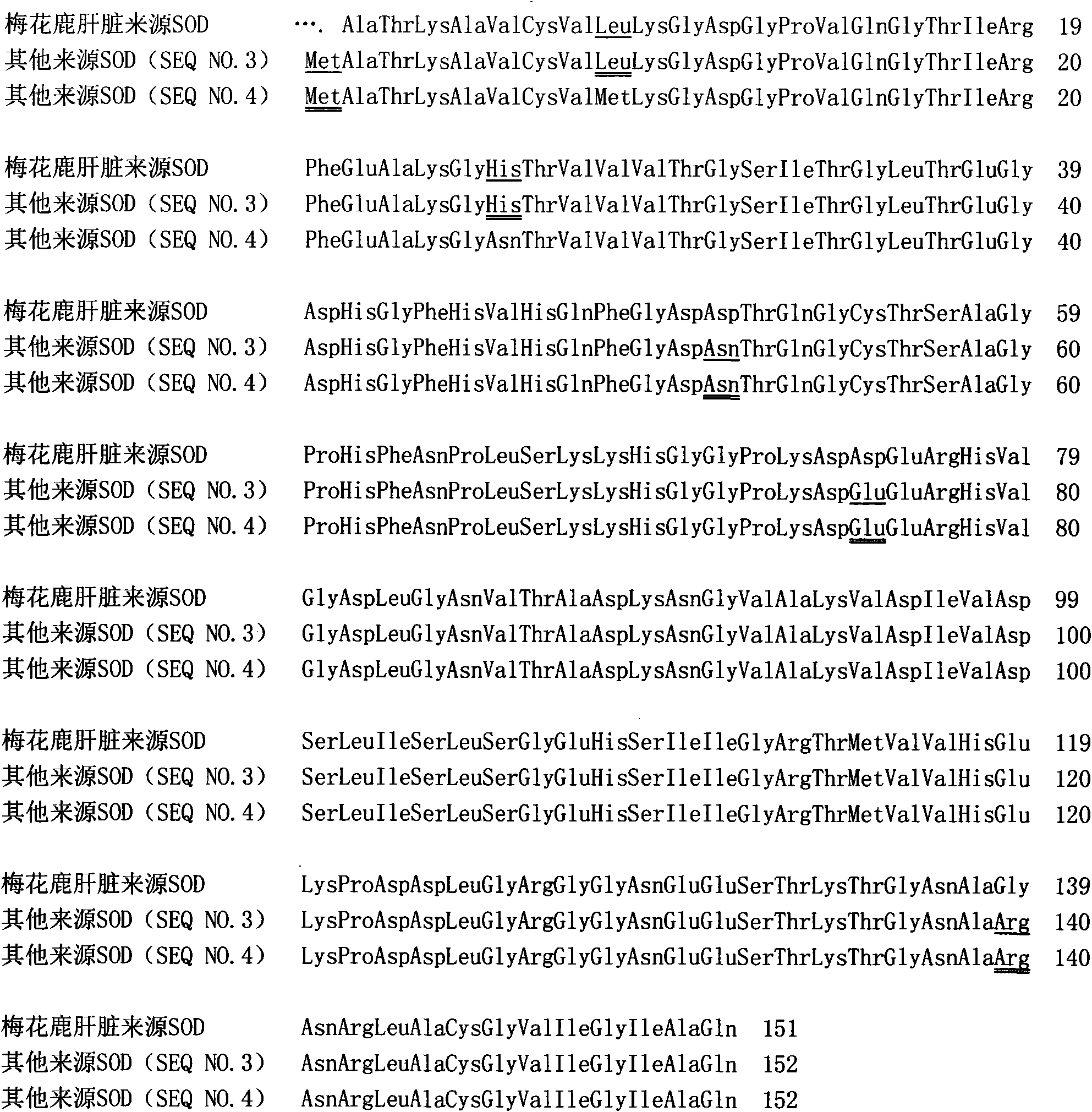
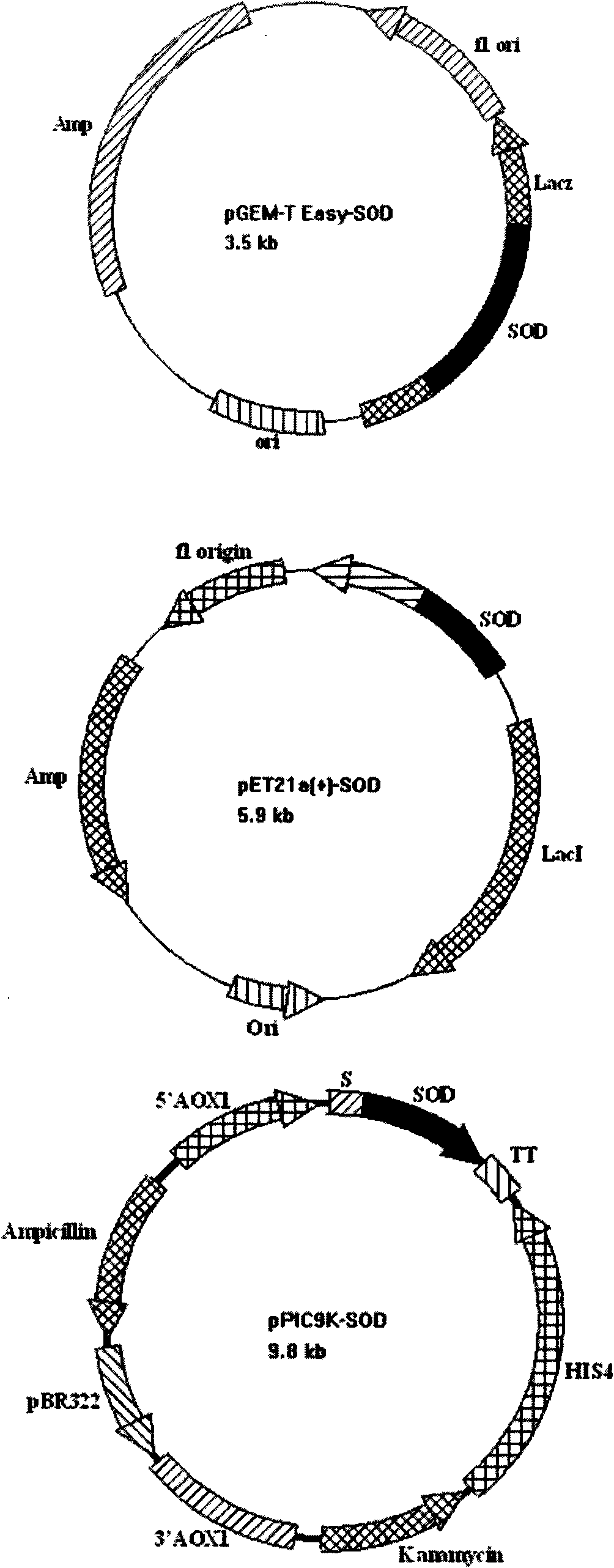
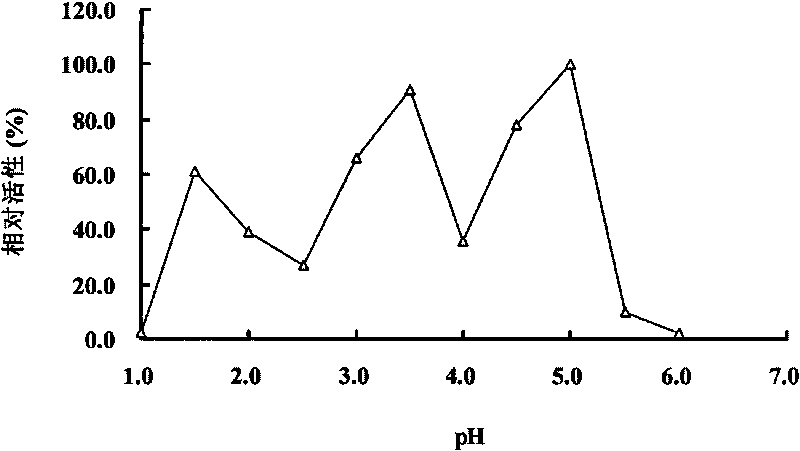
The invention provides a SOD gene from animal and a method for cloning and expressing the SOD gene in colibacillus and yeast cells, wherein a nucleotide sequence of superoxide dismutase is shown as SEQ NO.1; an amino acid sequence of the superoxide dismutase is shown as SEQ NO.2; carriers of nucleotide molecules are colibacillus plasmids or yeast plasmids; cells of the nucleotide molecules are formed by carrier conversion; and cells of the nucleotide molecules of the superoxide dismutase contain colibacillus containing the nucleotide molecules or converted by the carriers or pichia yeast containing the nucleotide molecules or converted by the carriers. The invention can prepare recombinant production strains which can efficiently express and secrete Cu / Zn-SOD, realizes the production industrialization of the Cu / Zn-SOD, and achieves good pH stability, favorable thermal stability and Cu / Zn-SOD products with anti-protease hydrolyzation capacity.
Owner:FUZHOU UNIV
Acidophil Beta-glucanase GLU7A and gene and application thereof
ActiveCN101748108AGood substrate adaptabilityImprove digestive energyFungiBacteriaBiotechnologyCellulose
The invention relates to the field of genetic engineering, in particular to an acidophil Beta-glucanase GLU7A and gene and application thereof. The invention provides a glucanase GLU7A from acidophil bispora Bisporasp, and the amino acid sequence thereof is shown as SEQIDNO.1, and the invention provides a genome of coding the glucanase and cDNA coding gene glu7A. The invention obtains an acidophil glucanase which has the optimum pH value of 1.5-5.0, simultaneously remains high enzymatic activity in acid environment, and resists protease hydrolysis. In addition, the glucanase not only hydrolyzes Beta-1, 3-1, 4-glucanase, but also can hydrolyze cellulose. Due to the properties, the acidophil Beta-glucanase GLU7A can be applied in the industries of food, feedstuff and beer.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Antibiotic, Compositions Containing the Antibiotic, and Methods for Administering the Antibiotic and/or Said Compositions to Livestock
InactiveUS20070009503A1Growth inhibitionEfficient growth processAntibacterial agentsBiocideBacteroidesBiotechnology
An antibiotic comprising a protease-resistant bacteriocin derived from a lactic acid bacterium, and compositions thereof, are disclosed. A feed composition for livestock comprising the antibiotic comprising a protease-resistant bacteriocin derived from a lactic acid bacterium is also disclosed. A method for preventing the growth of human food poisoning-causing bacteria in the stomach and / or intestines of livestock comprising administering the feed composition comprising a protease-resistant bacteriocin derived from a lactic acid bacterium to livestock is disclosed.
Owner:AJINOMOTO CO INC
Method for producing lactic acid bacterium culture containing bacteriocin and a method for preserving food products for by using it
InactiveUS20060270019A1BacteriaMicrobiological testing/measurementBiotechnologyLactic acid bacterium
The present invention provides a lactic acid bacterium culture containing protease-resistant bacteriocin, which can be produced by cultivating lactic acid bacteria (e.g., from the genus Weissella). As such, the shelf stability of a food can be improved by incorporating the culture of the present invention therein.
Owner:AJINOMOTO CO INC
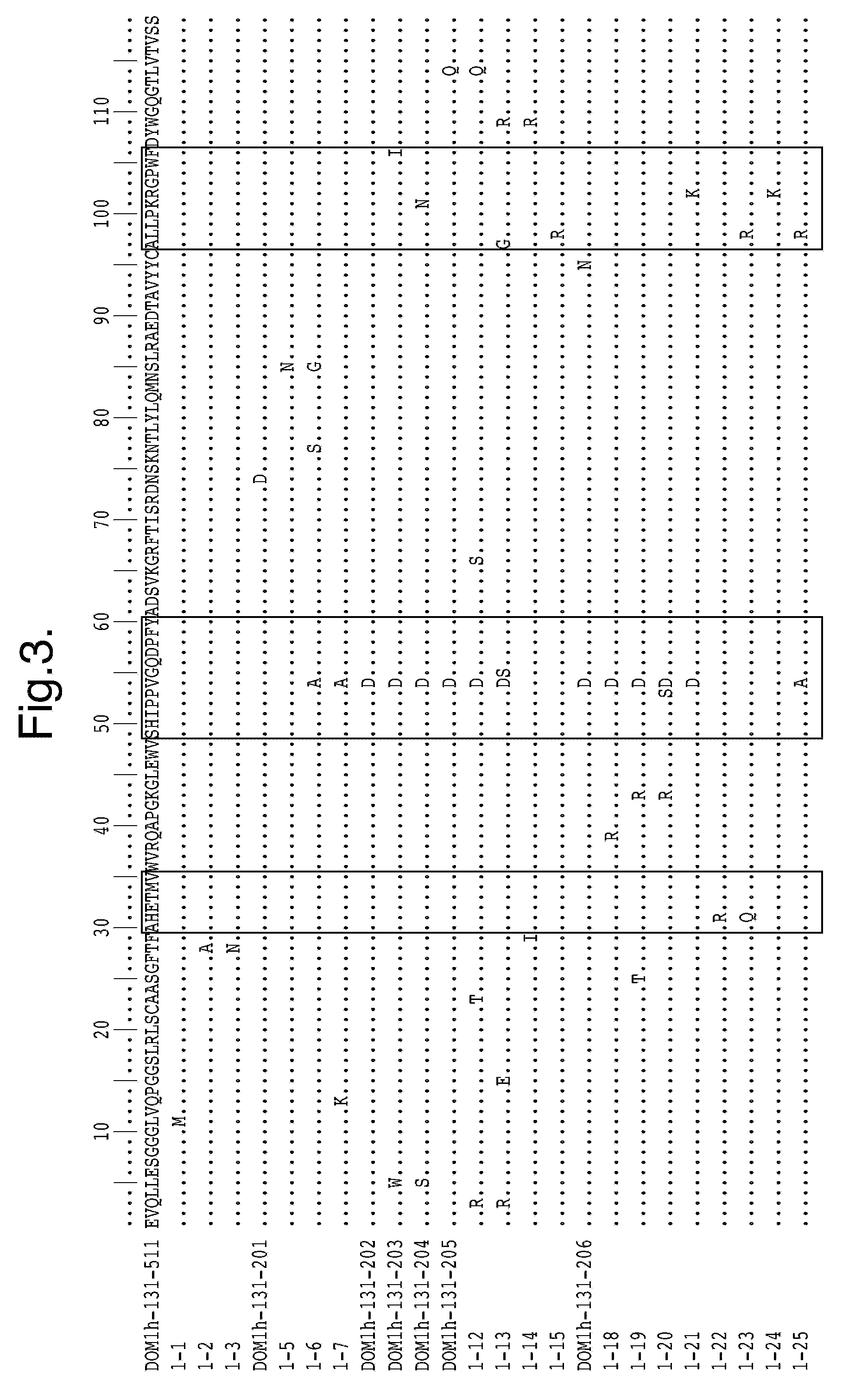
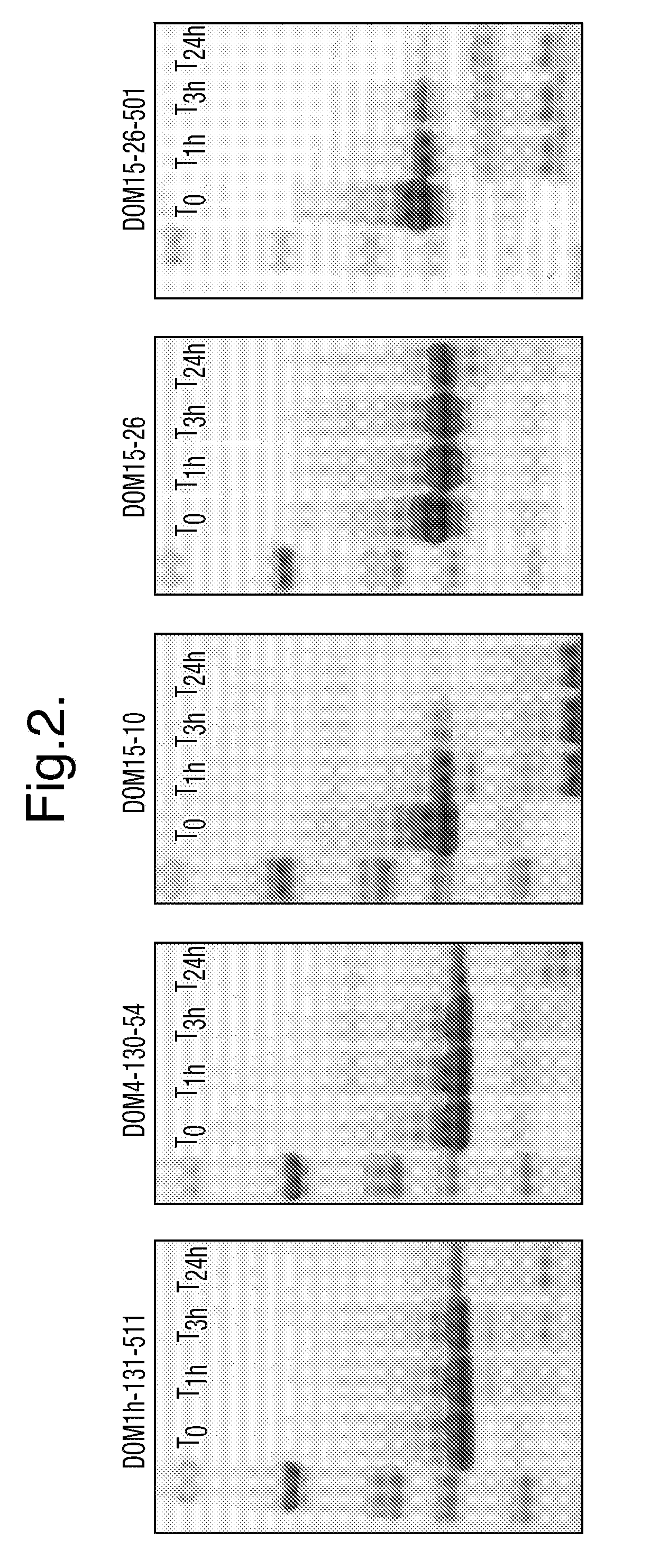
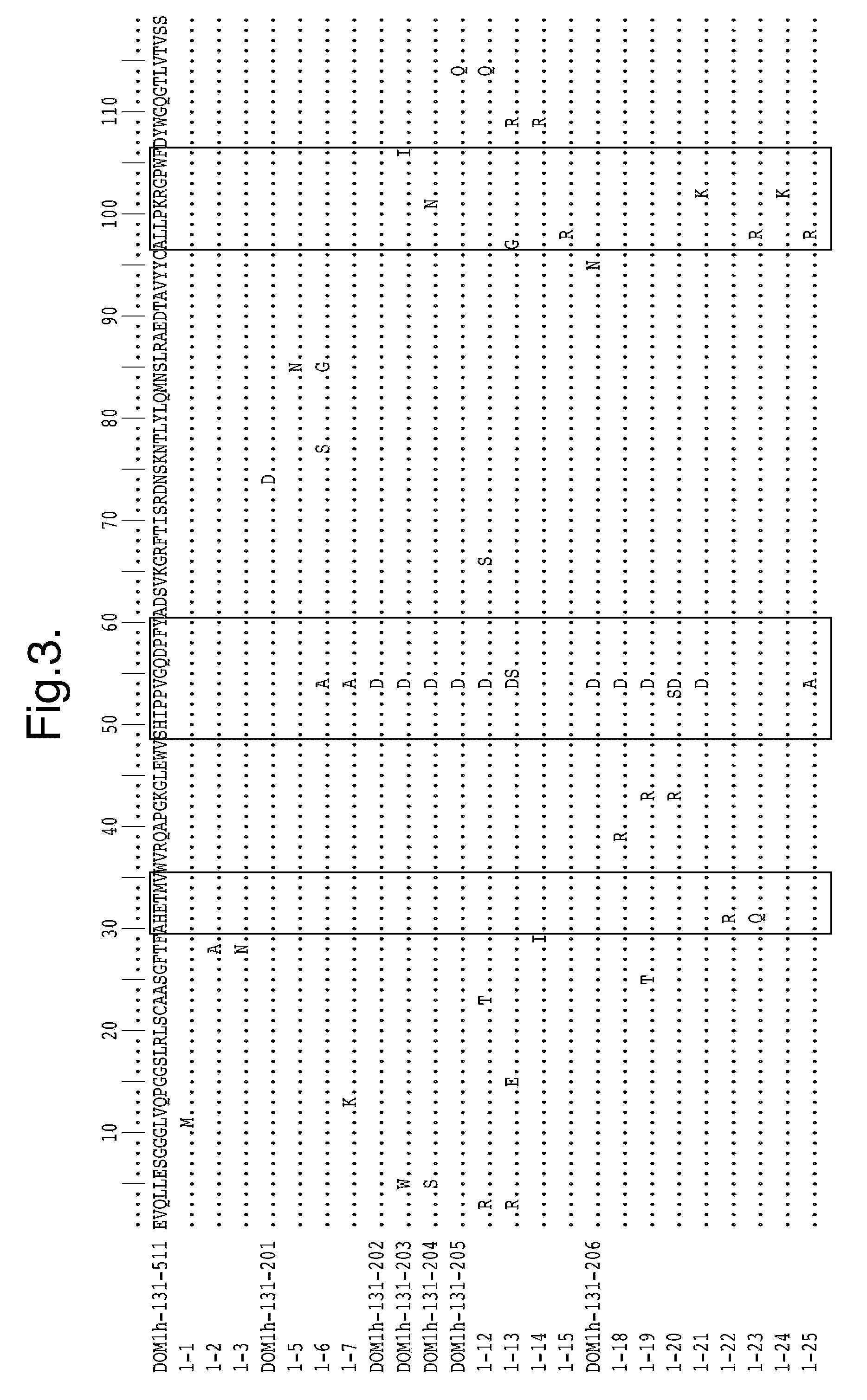
Polypeptides, antibody variable domains and antagonists
InactiveUS20100254995A1Increase temperatureImprove stabilityAntibacterial agentsSenses disorderDiseaseArthritis
The invention relates to anti-VEGF polypeptides and antibody single variable domains (dAbs) that are resistant to degradation by a protease, as well as antagonists comprising these. The polypeptides, dAbs and antagonists are useful for pulmonary administration, oral administration, delivery to the lung and delivery to the GI tract of a patient, as well as for treating cancer and inflammatory disease, such as arthritis.
Owner:DORMANTIS LTD
Methods for selecting protease resistant polypeptides
InactiveUS20100254972A1Therapeutic utilityIncrease temperatureAntibacterial agentsSenses disorderDiseaseProteinase activity
The invention relates to a method for selecting, isolating and / or recovering a peptide or polypeptide from a library or a repertoire of peptides and polypeptides (e.g., a display system) that is resistant to degradation by a protease such as a protease found in the GI tract or pulmonary tissue of a human. Generally, the method comprises providing a library or repertoire of peptides or polypeptides, combining the library or repertoire with a protease under conditions suitable for protease activity, and selecting, isolating and / or recovering a peptide or polypeptide that is resistant to degradation by the protease and has a desired biological activity. The selected peptides and polypeptides have utility as therapeutics, eg for treating disease or conditions of GI tract or pulmonary tissue in humans.
Owner:DORMANTIS LTD
Anti-protease acidic alpha-galactosidase Aga-F75 and gene and application thereof
ActiveCN101457208AAppropriate pH valueStrong protease resistanceFungiBacteriaFood additiveBiotechnology
The invention relates to the genetic engineering field, especially to a novel gibberella Gibberela.sp. F75 with a preservation number CGMCC No.2499, and an acidic alpha-galactosidase Aga-F75 of antiprotease from said strain, further a gene thereof and a recombinant vector containing said gene. The amino acid sequence of the alpha-galactosidase Aga-F75 is shown as SEQ ID NO.1. The enzyme has a proper action pH value, a stronger protease resistance and a better hydrolysis ability for various substrates and is used for the feed and food industries as a feed or food additive agent.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
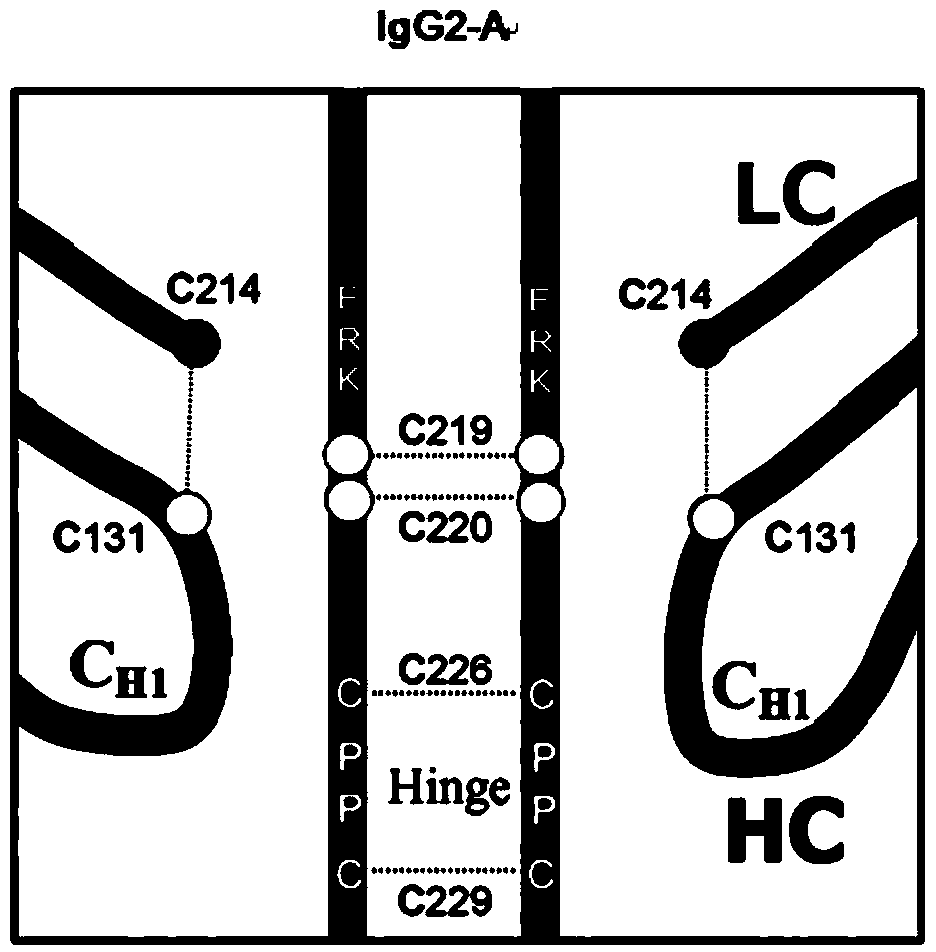
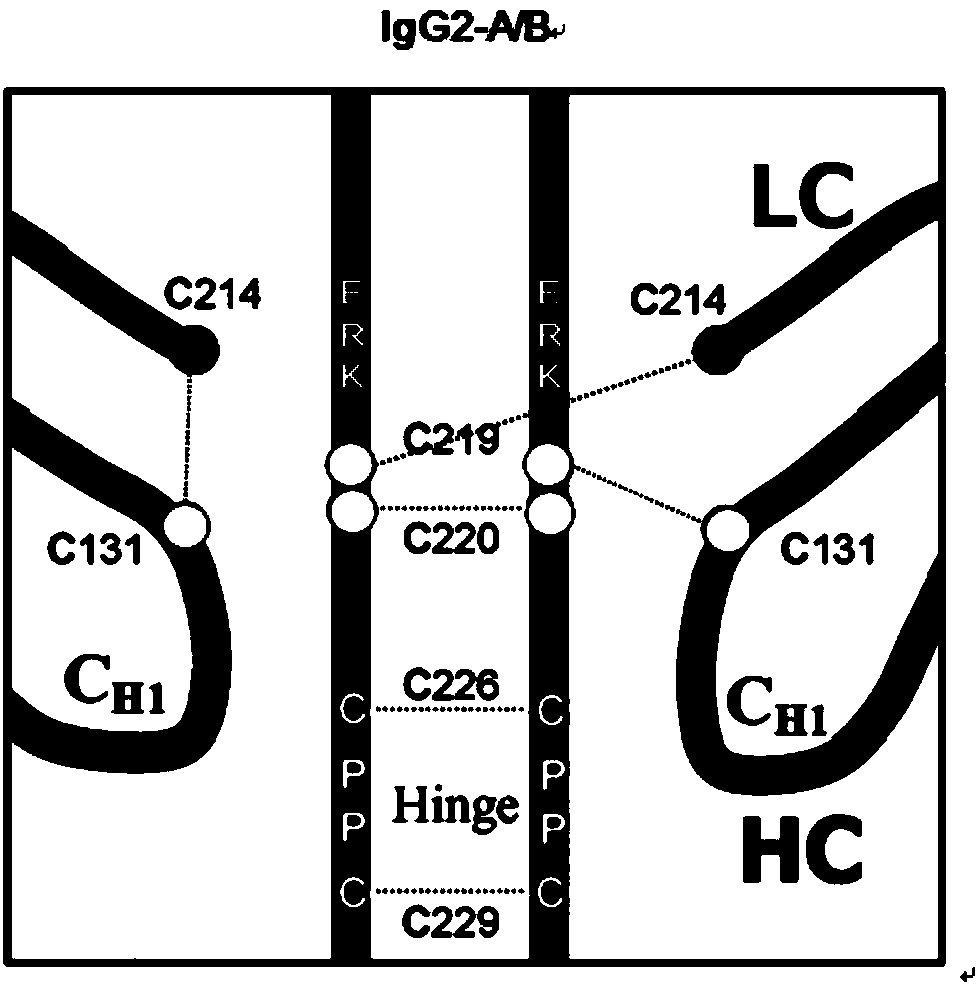
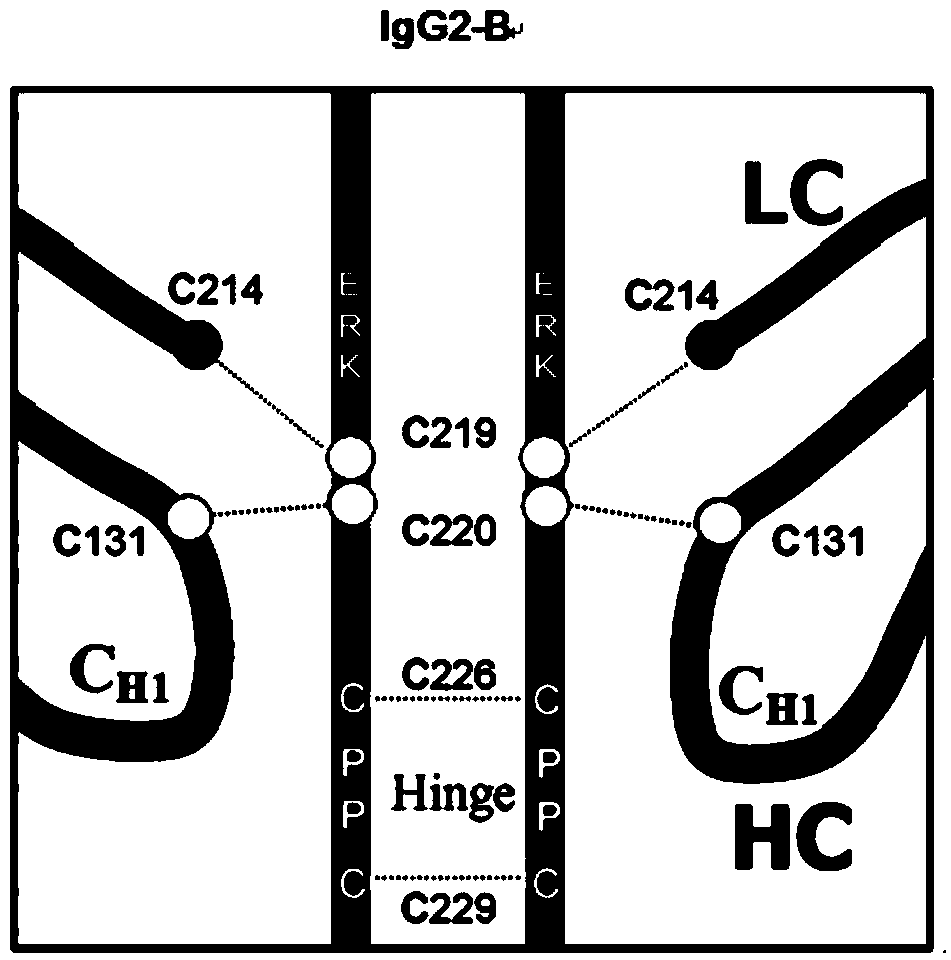
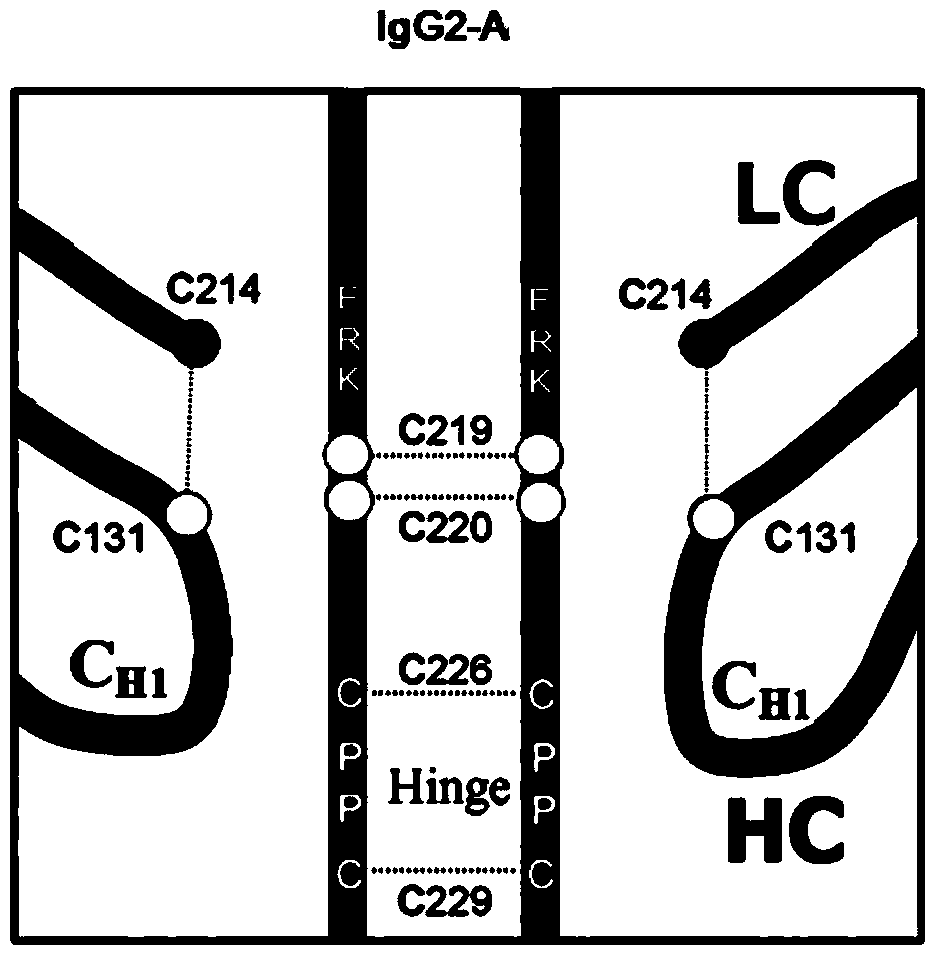
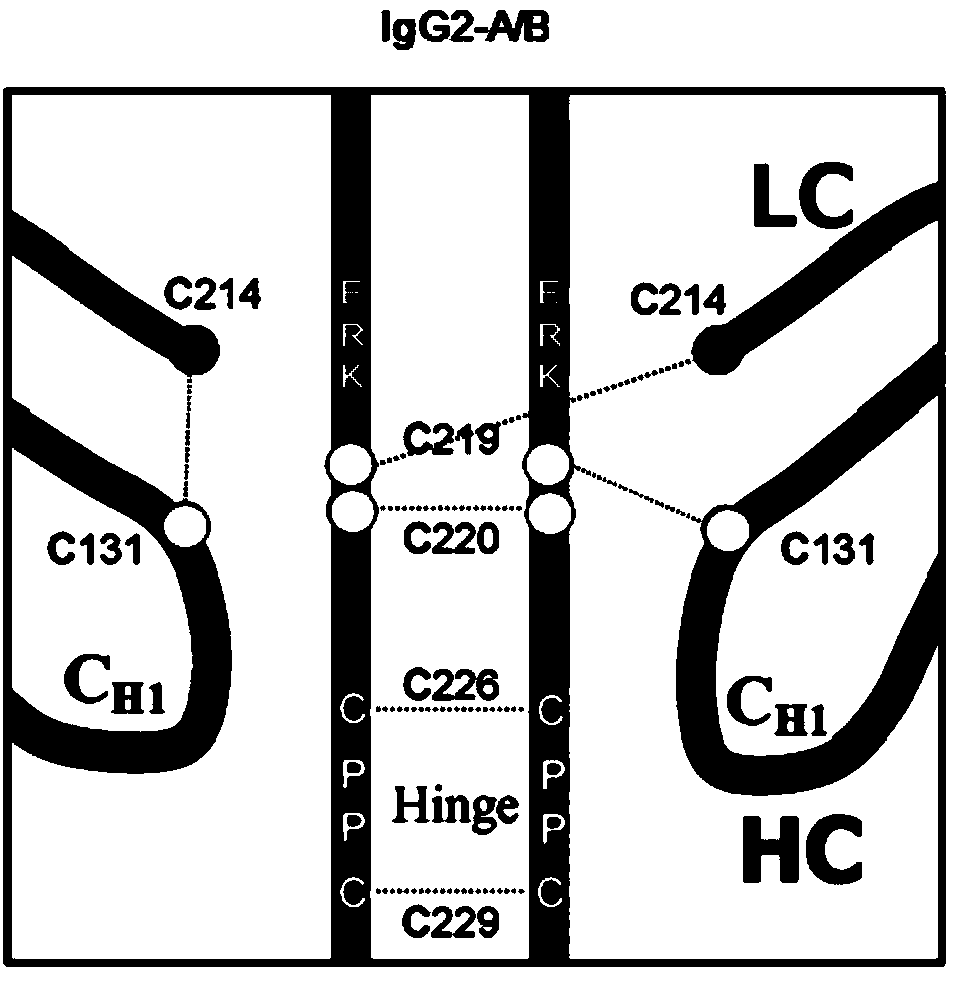
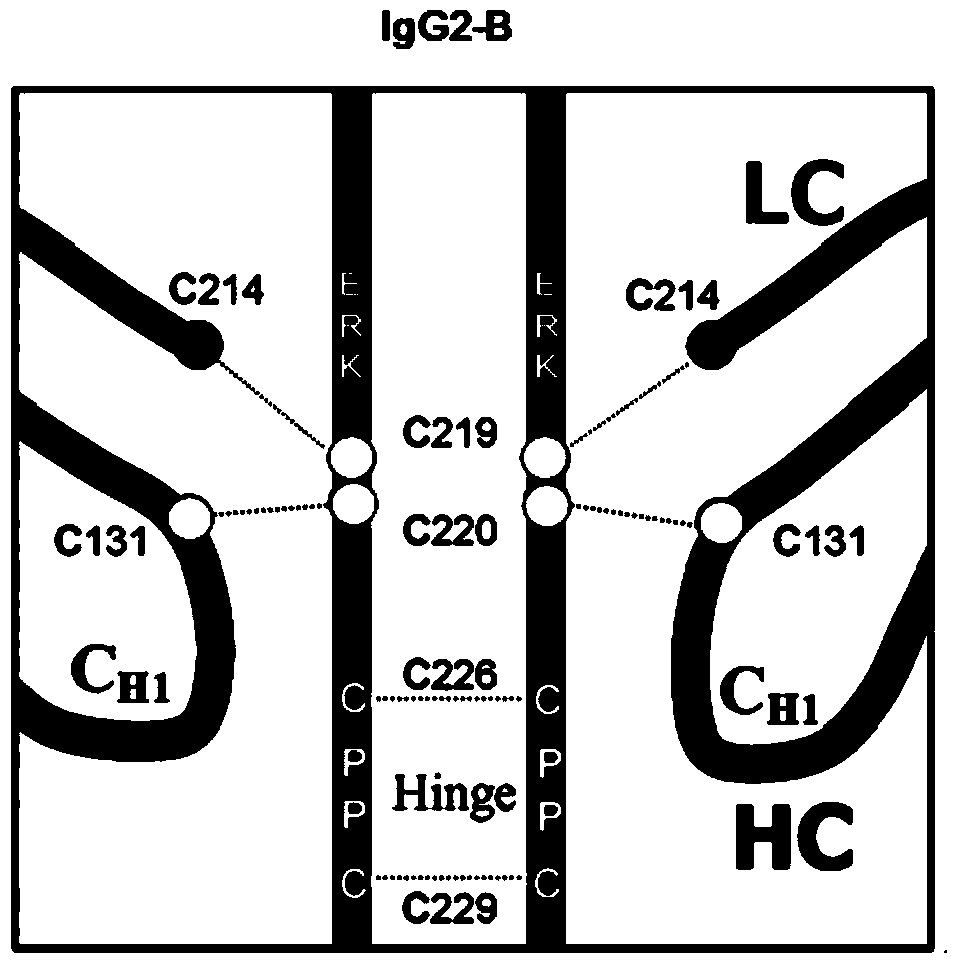
Hinge region modification body of human IgG2 antibody
ActiveCN104177496BImprove hydrolysis effectImmunoglobulins against animals/humansSkeletal disorderHeavy chainHinge region
The invention discloses a hinge region modified recombinant human IgG2 antibody. Amino acids at the sites of Glu216Arg217Lys218 are deleted in a heavy chain hinge region of the antibody, and amino acid is substituted and / or deleted at the site of Cys219 and / or Cys220. The invention further provides a method for improving the antiprotease hydrolysis effect of the recombinant human IgG2 antibody. According to the IgG2 antibody provided by the invention, the heterogeneity caused by disulfide bonds in the hinge region of the antibody is eliminated, and the effect of improving the antiprotease hydrolysis effect is achieved.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Methods of treatment for meconium aspiration syndrome
InactiveUS20070292407A1Low viscosityReduce thicknessBiocidePeptide/protein ingredientsProphylactic treatmentAcetylcysteine
The present invention relates to compositions and methods relating to prophylactic treatment and treatment of meconium aspiration syndrome comprising administering to a subject in need of treatment a therapeutically effective amount of acetylcysteine (N-acetyl-L-cysteine). One aspect of the invention relates to pharmaceutical compositions and methods of treatment comprising acetylcysteine and / or DNAse and / or a compound of the antiprotease family of drugs, administered singly or in combination, as medicament for treatment of meconium aspiration syndrome admixed with a pharmaceutically acceptable diluent, carrier, or excipient.
Owner:BOARD OF TRUSTEES OPERATING MICHIGAN STATE UNIV
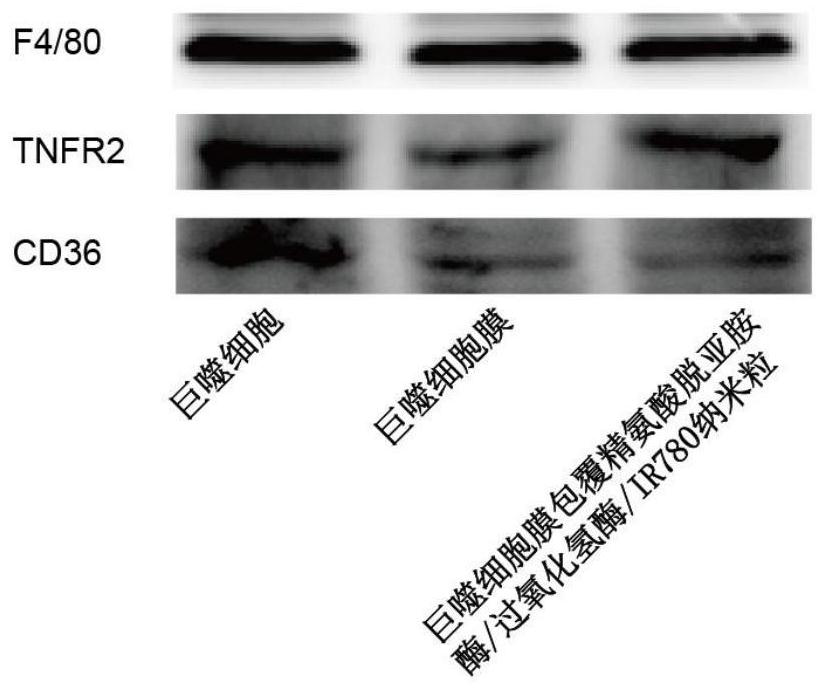
Macrophage membrane coated arginine deiminase/catalase/IR780 nanoparticle, preparation method and application
ActiveCN113750232AImprove stabilityLow immunogenicityPeptide/protein ingredientsHydrolasesCell membraneCatalase
The invention belongs to the field of pharmaceutical preparations. The invention relates to a preparation method and application of a macrophage membrane coated arginine deiminase / catalase / IR780 nanoparticle. The prepared macrophage membrane coated arginine deiminase / catalase / IR780 nanoparticle is good in biocompatibility, the preparation method is simple, the particle size distribution of the nanoparticle is uniform, the stability of the arginine deiminase can be improved, the protease hydrolysis resistance ability of the arginine deiminase is improved, an anti-tumor effect is enhanced in cooperation with photothermal therapy or photodynamic therapy, and a potential application value is achieved in the fields of treatment of cancers (especially arginine-dependent diseases) and the like.
Owner:CHONGQING MEDICAL UNIVERSITY
Lactobacillus acidophilus nucleic acid sequences encoding protease homologues and uses therefore
InactiveUS7455992B2Improve stabilityMilk preparationSugar derivativesAntigenAntiendomysial antibodies
Protease-like nucleic acid molecules and polypeptides and fragments and variants thereof are disclosed in the current invention. In addition, protease-like fusion proteins, antigenic peptides, and anti-protease-like antibodies are encompassed. The invention also provides vectors containing a nucleic acid molecule of the invention and cells into which the vectors have been introduced. Methods for producing the polypeptides and methods of use for the polypeptides of the invention are further disclosed.
Owner:NORTH CAROLINA STATE UNIV
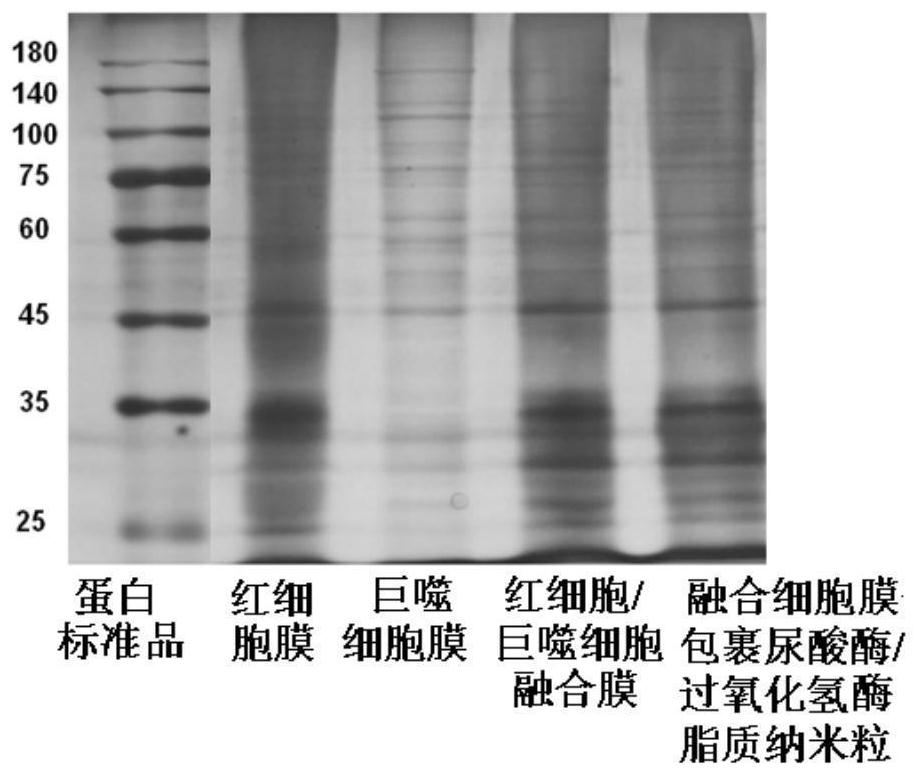
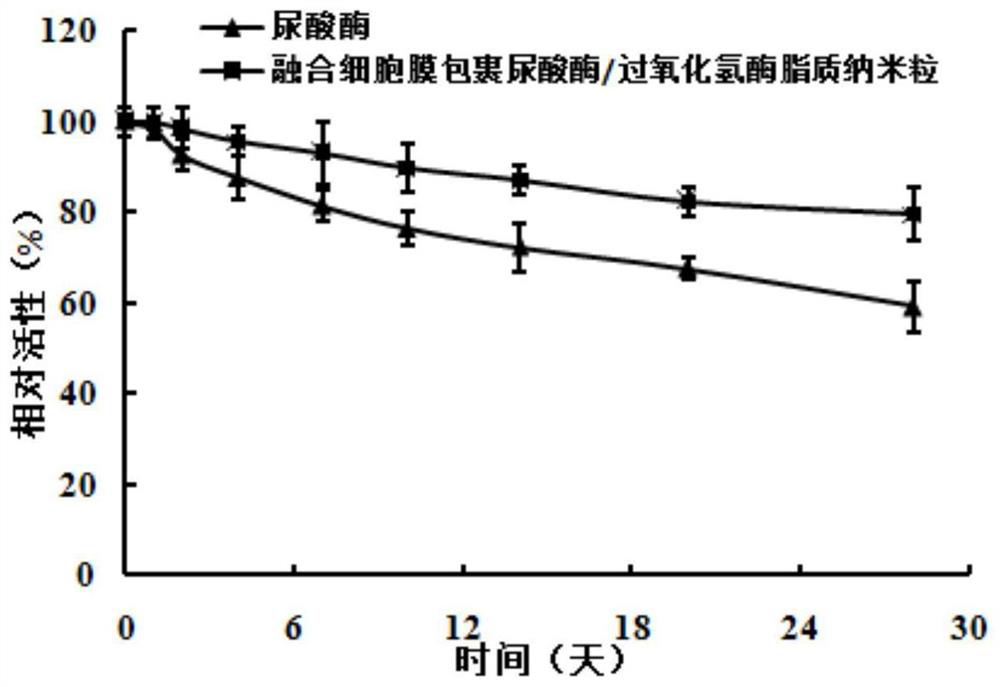
Uricase/catalase lipid nanoparticles wrapped by fusion cell membrane and preparation method of uricase/catalase lipid nanoparticles
ActiveCN114306581AImprove stabilityReduced immune activityPeptide/protein ingredientsSkeletal disorderNanoparticleCell membrane
The invention belongs to the field of pharmaceutical preparations. The invention relates to a uricase / catalase lipid nanoparticle wrapped by a fusion cell membrane and a preparation method of the uricase / catalase lipid nanoparticle. According to the prepared fusion cell membrane coated uricase / catalase lipid nanoparticles, the stability of uricase can be improved, the protease hydrolysis resistance of uricase is improved, the bioavailability is improved, the inflammation chemotactic ability is enhanced, and the toxic and side effects are reduced.
Owner:CHONGQING MEDICAL UNIVERSITY
Antimicrobial peptide, preparation method of antimicrobial peptide, antimicrobial composition, antibacterial method and applications
ActiveCN109535227AHigh purityIntegrity guaranteedAntibacterial agentsPeptide/protein ingredientsChemical synthesisAntibacterial activity
The present invention relates to an antimicrobial peptide, a preparation method of the antimicrobial peptide, a composition containing the antimicrobial peptide, an antibacterial method using the antimicrobial peptide, and applications of the antimicrobial peptide. The amino acid sequence of the antimicrobial peptide is Val Pro Tyr Ile Gln His Thr Pro Asn Leu Leu Leu Glu Gln Asn LeuLeu, wherein amino acids in the amino acid sequence are all D-type amino acids. In the case, chemical synthesis is used to efficiently obtain a large amount of high-purity total reverse D-type antibacterial peptideso as to ensure the integrity and purity of the antibacterial peptide sequence, and the total reverse D-type antibacterial peptide has high thermal stability and anti-protease degradation ability. Thebroad-spectrum antibacterial activity of the total reverse D-type antibacterial peptide in this case is greatly enhanced compared with L-type antibacterial peptides.
Owner:XIAN CHILDRENS HOSPITAL
Lactobacillus acidophilus nucleic acid sequences encoding protease homologues and uses therefore
InactiveUS20070003667A1Improve stabilityMilk preparationBacteriaProteinase activityNucleic acid sequencing
Protease-like nucleic acid molecules and polypeptides and fragments and variants thereof are disclosed in the current invention. In addition, protease-like fusion proteins, antigenic peptides, and anti-protease-like antibodies are encompassed. The invention also provides vectors containing a nucleic acid molecule of the invention and cells into which the vectors have been introduced. Methods for producing the polypeptides and methods of use for the polypeptides of the invention are further disclosed.
Owner:NORTH CAROLINA STATE UNIV
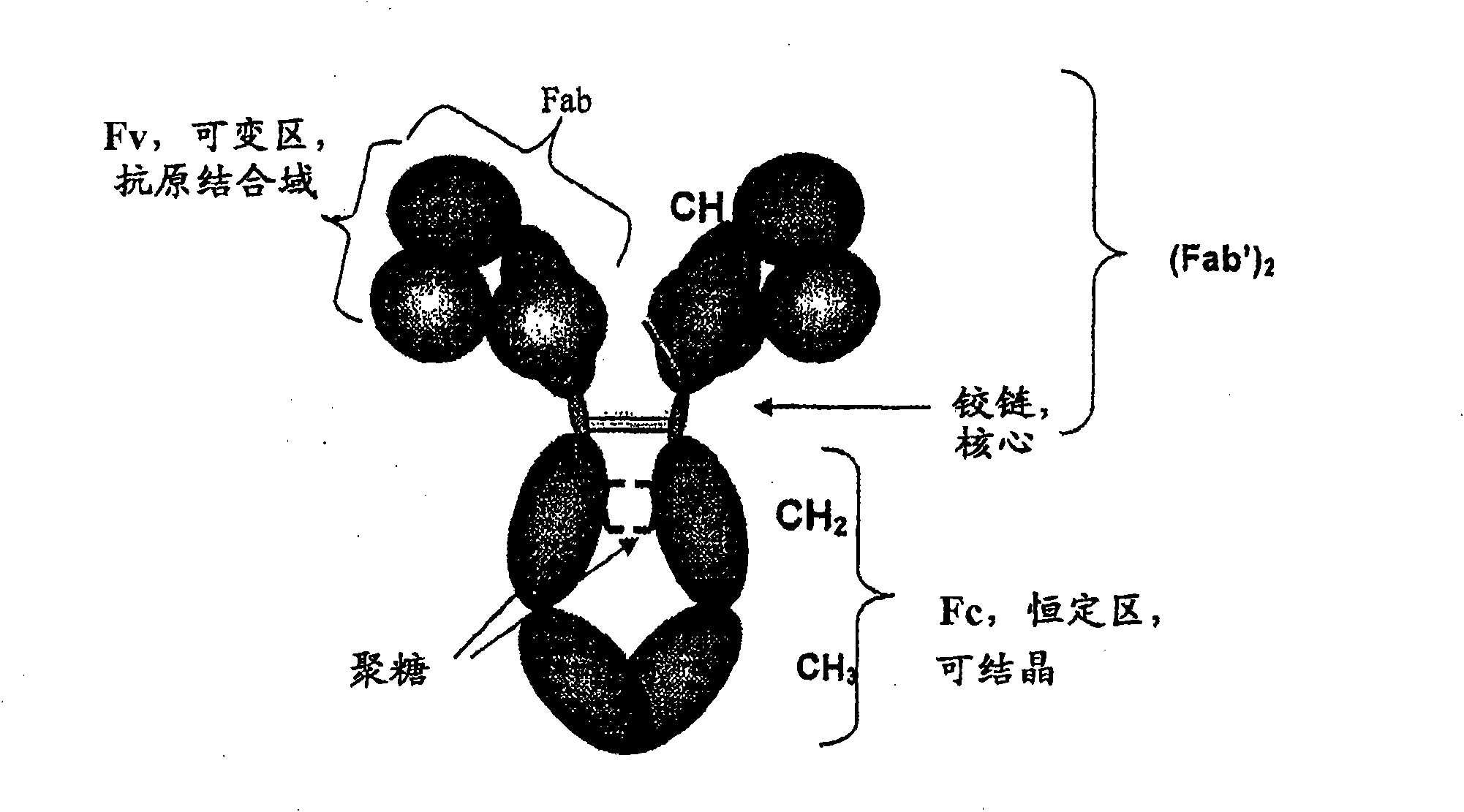
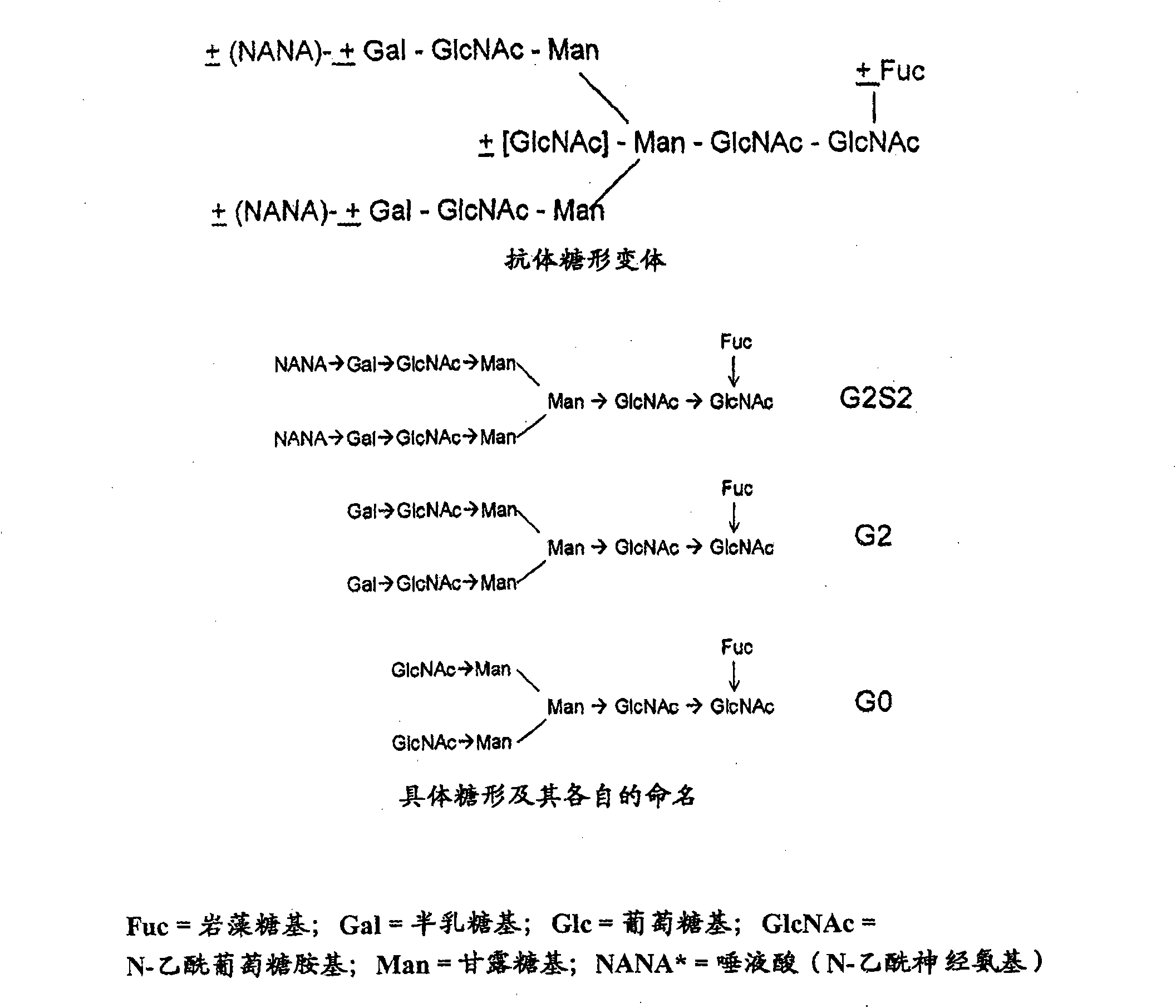
Proteolysis resistant antibody preparations
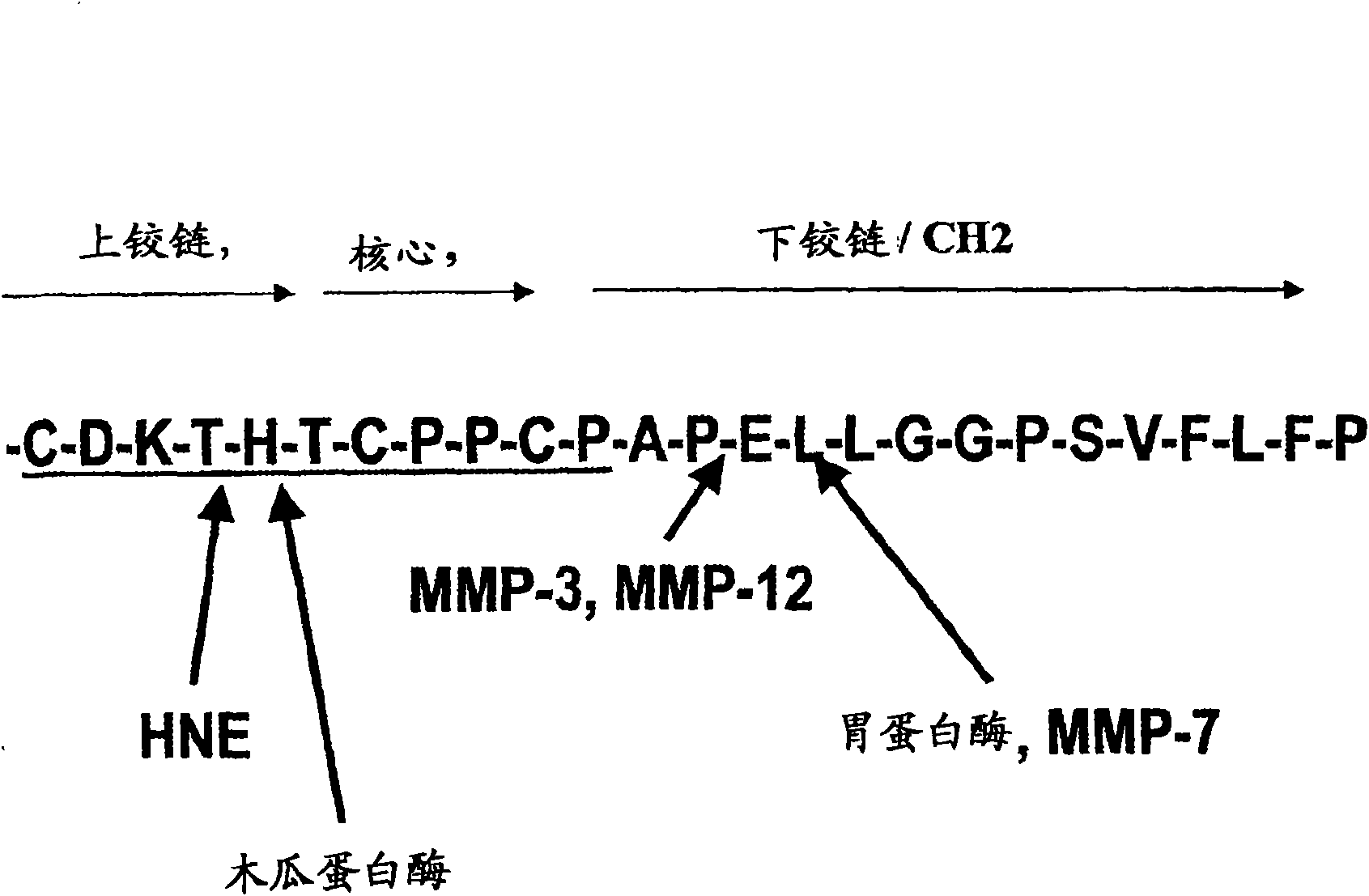
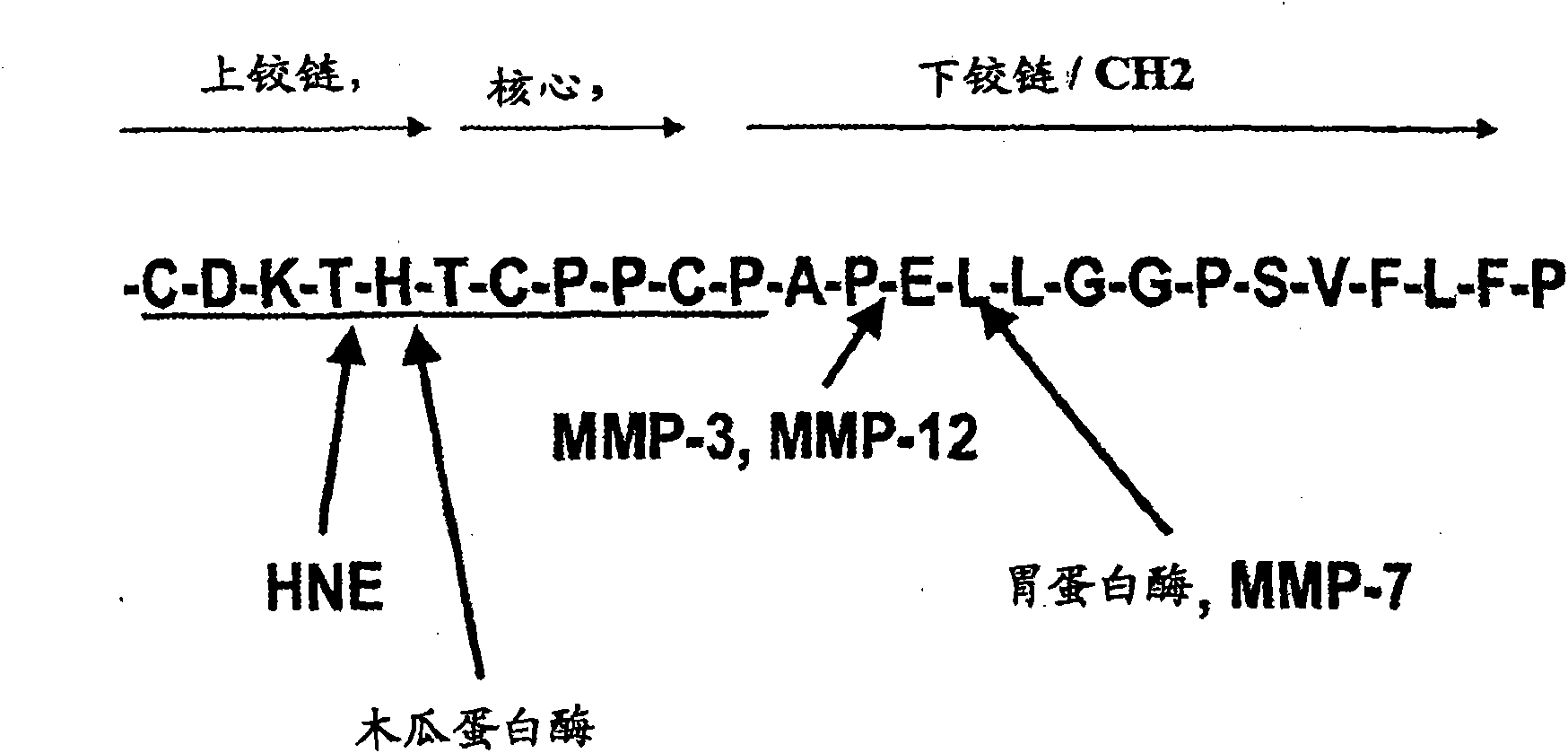
Antibody preparations with substantially homogeneous and unsialylated glycoforms, such as G0 and G2, are prepared by enzymatic treatment, expression under certain conditions, use of particular host cells, and contact with serum. These antibody preparations resist cleavage by proteases, such as papain, ficin, bromolein, pepsin, a matrix metalloproteinase, such as MMP-7, neutrophil elastase (HNE), stromelysin (MMP-3) and macrophage elastase (MMP-12), and glycosylation modification enzymes. The antibody preparations with substantially homogeneous and unsialylated glycoforms and methods of testing for glycosylation in an antibody are useful in connection with characterization of antibody properties and / or in diseases or conditions characterized by an increase in protease activity.
Owner:CENTOCOR
Conjugate of mutant protease 3 and biotin, and preparation method and application of conjugate
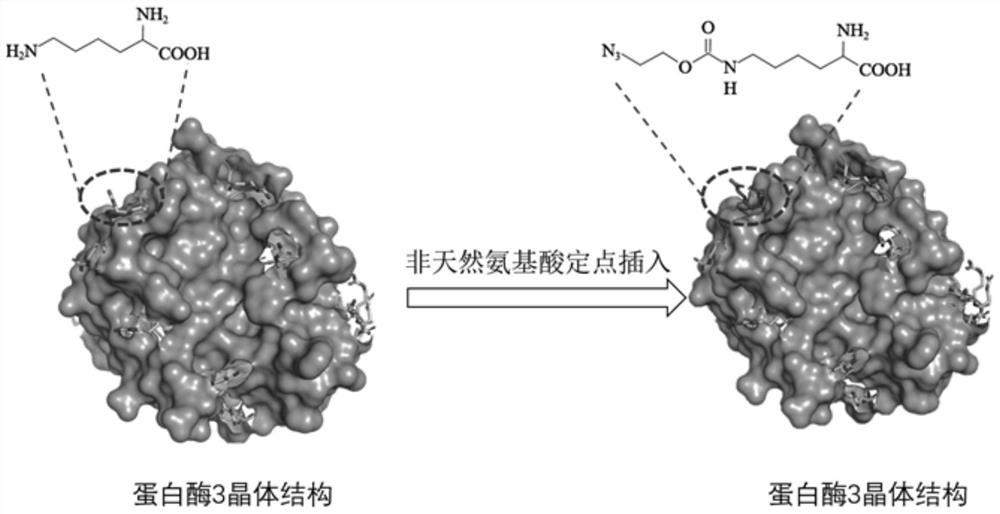
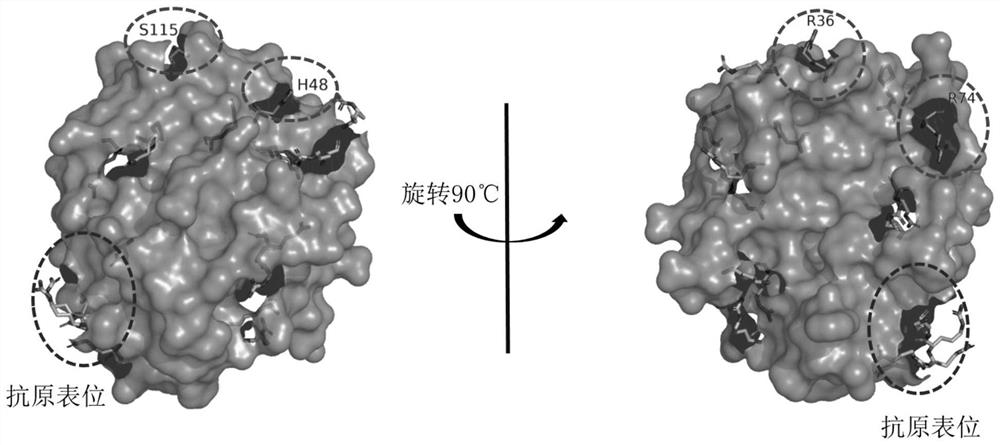
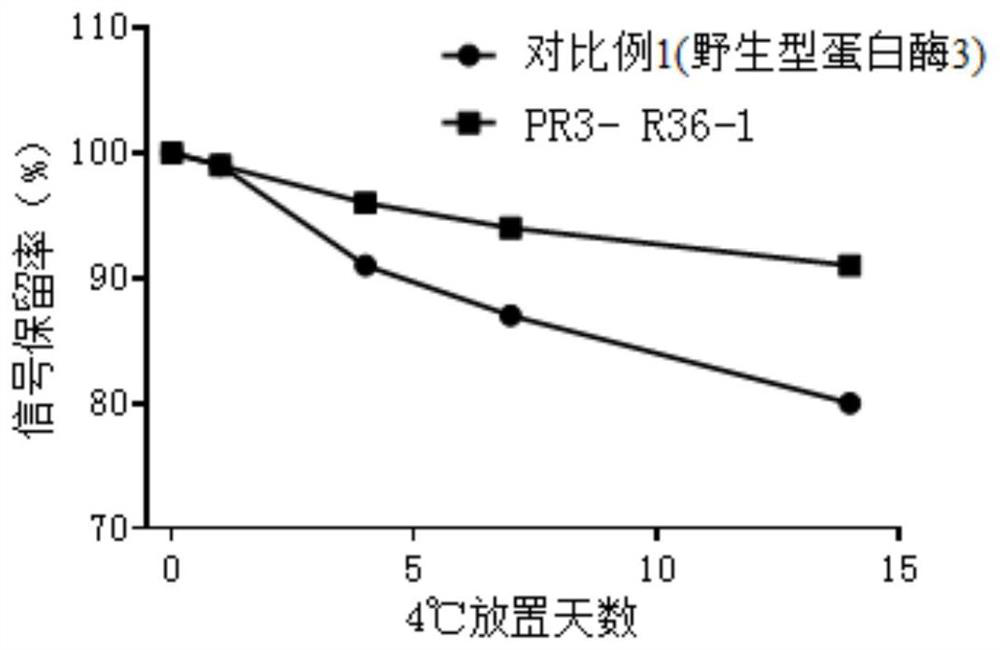
ActiveCN113969272AImprove labeling efficiencyAvoid shadowsHydrolasesChemiluminescene/bioluminescenceChemical reactionClick chemistry
The invention provides a conjugate of mutant protease 3 and biotin, and a preparation method and application of the conjugate. The amino acid at the 36 site, the 48 site or the 74 site of the amino acid sequence of the mutant protease 3 is mutated into non-natural amino acid with one of an azide group and an alkynyl group; the biotin is a biotin derivative with the other one of the alkynyl group and the azide group; and the conjugate of the mutant protease 3 and the biotin is obtained by carrying out a click chemical reaction on the non-natural amino acid and the biotin derivative. The invention has the advantages of strong operability, high reaction efficiency and mild conditions, improves the biotin labeling efficiency, enhances the antigen-antibody binding reactivity, improves the sensitivity and accuracy of antiproteinase 3 antibody detection, and has wide prospects in clinical application.
Owner:苏州携创生物技术有限公司
Anti-protease 3 antibody IgG chemiluminiscence immunodetection kit and preparation method thereof
InactiveCN106501506ALow costHigh detection sensitivityMaterial analysisProteinase activityIgG.monoclonal
The invention discloses an anti-protease 3 antibody IgG chemiluminiscence immunodetection kit. The kit comprises magnetic particles coated with a protease 3 antibody, acridinium ester coated with a mouse anti-human IgG monoclonal antibody, an anti-protease 3 antibody IgG calibration product, a pre-exciting solution and an exciting solution. The invention further discloses a preparation method of the anti-protease 3 antibody IgG chemiluminiscence immunodetection kit. Compared with an existing kit, the kit has the advantages of being easy and convenient to operate, high in sensitivity, wide in detection range and the like.
Owner:SHENZHEN YHLO BIOTECH
Histone deacetylase and proteasome double-target inhibitor, and preparation method and application thereof
The invention relates to a protein deacetylase and proteasome double-target inhibitor, and a pharmaceutically acceptable salt, a stereoisomer, a preparation method and an application thereof. The compound is represented by general formula I, the compound has good histone deacetylase resisting activity, proteasome resisting activity and tumor cell proliferation resisting activity, and can be used for preparing medicines for preventing or treating related mammalian diseases caused by abnormal histone deacetylase expression or proteasome abnormality. The invention also relates to a pharmaceuticalapplication of a composition containing the compound with the structure of general formula I.
Owner:SHANDONG UNIV
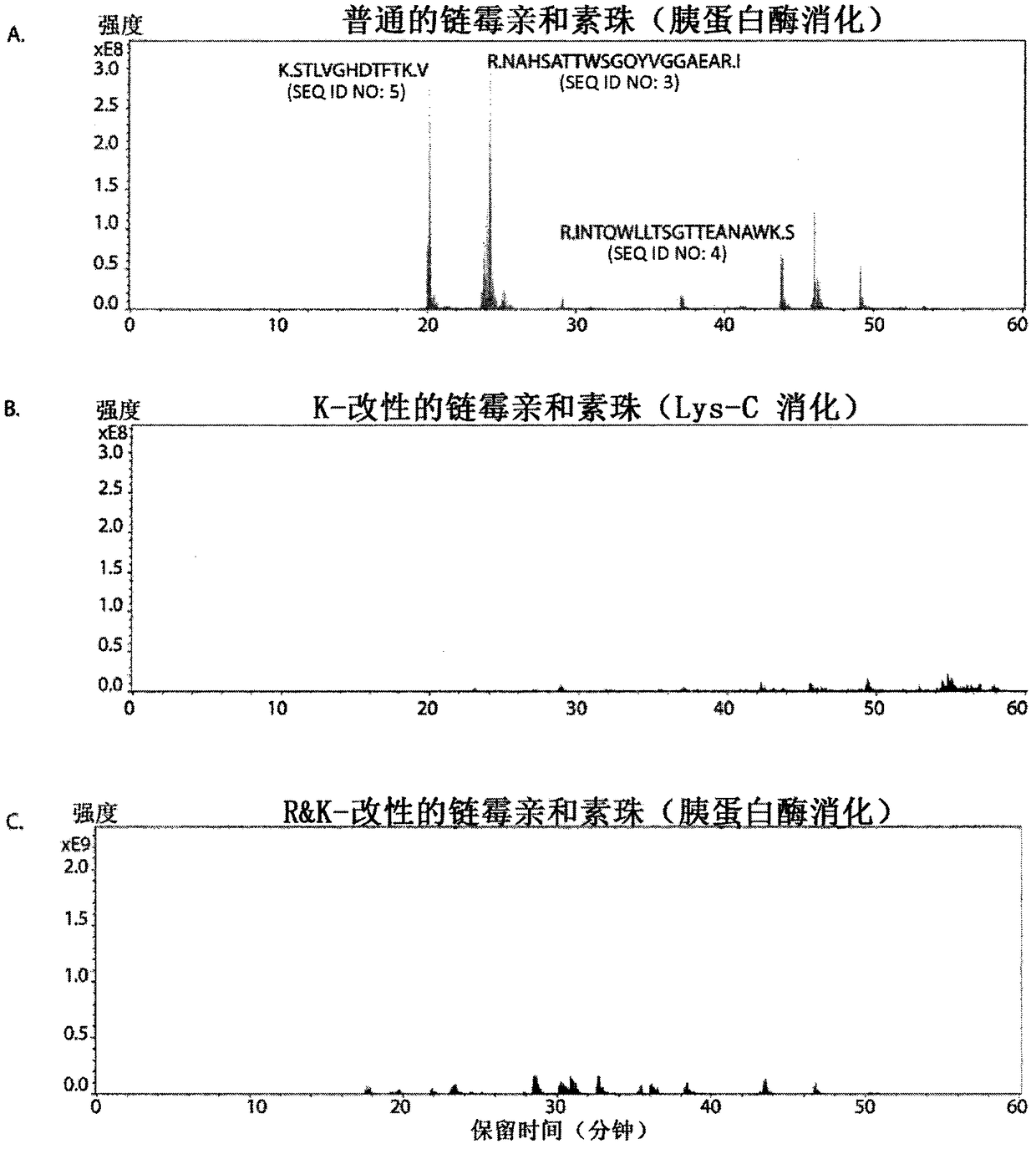
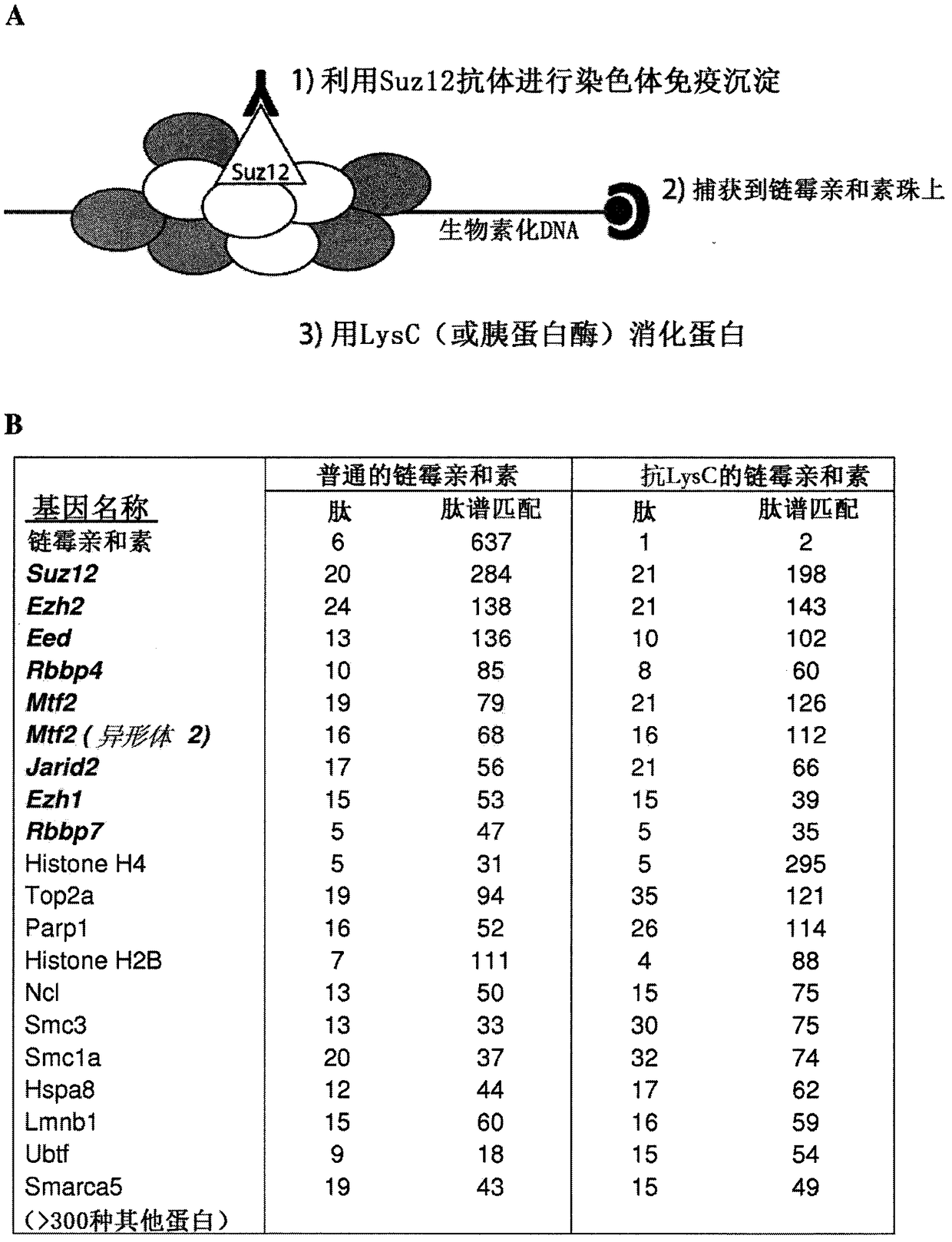
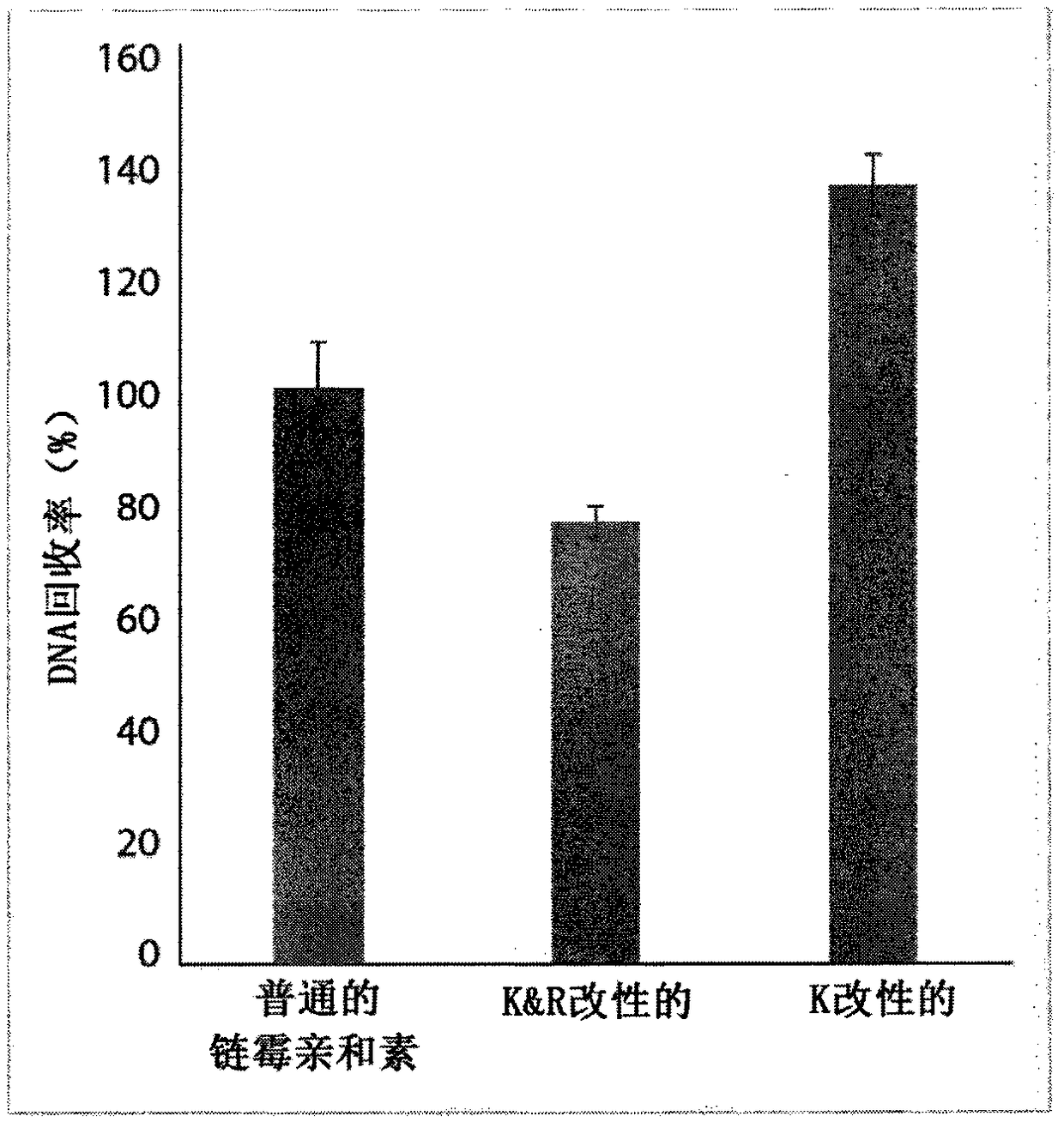
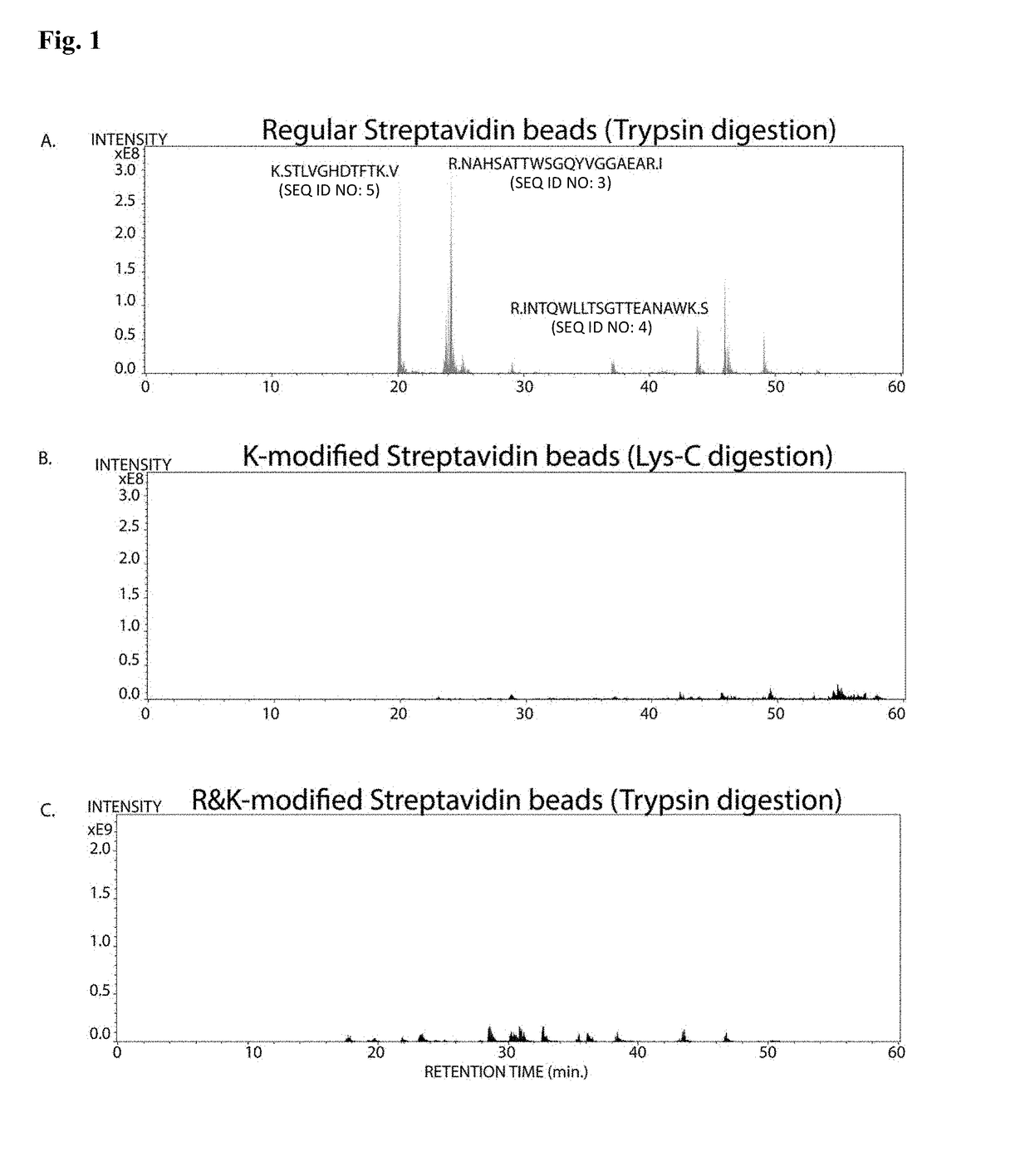
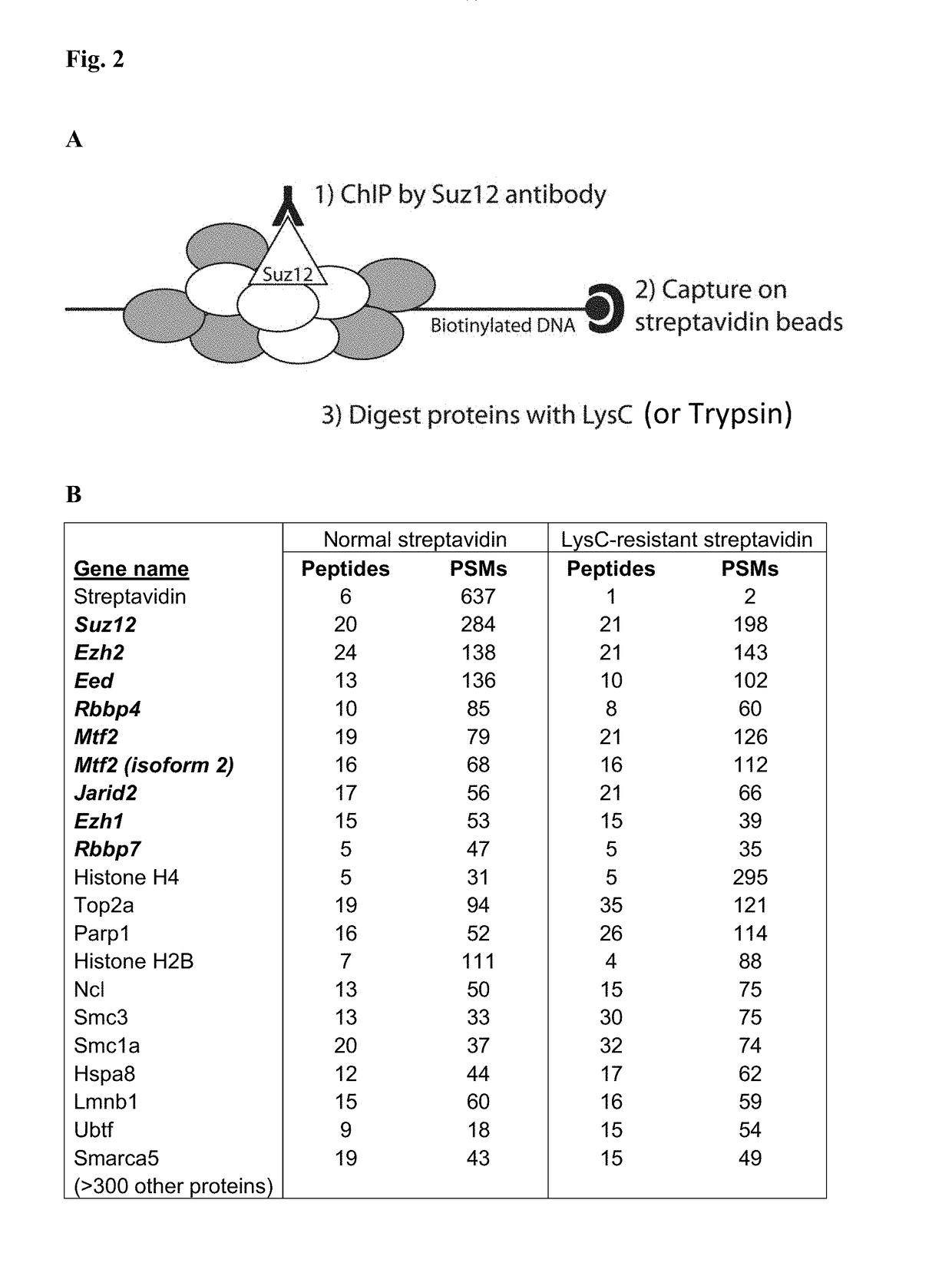
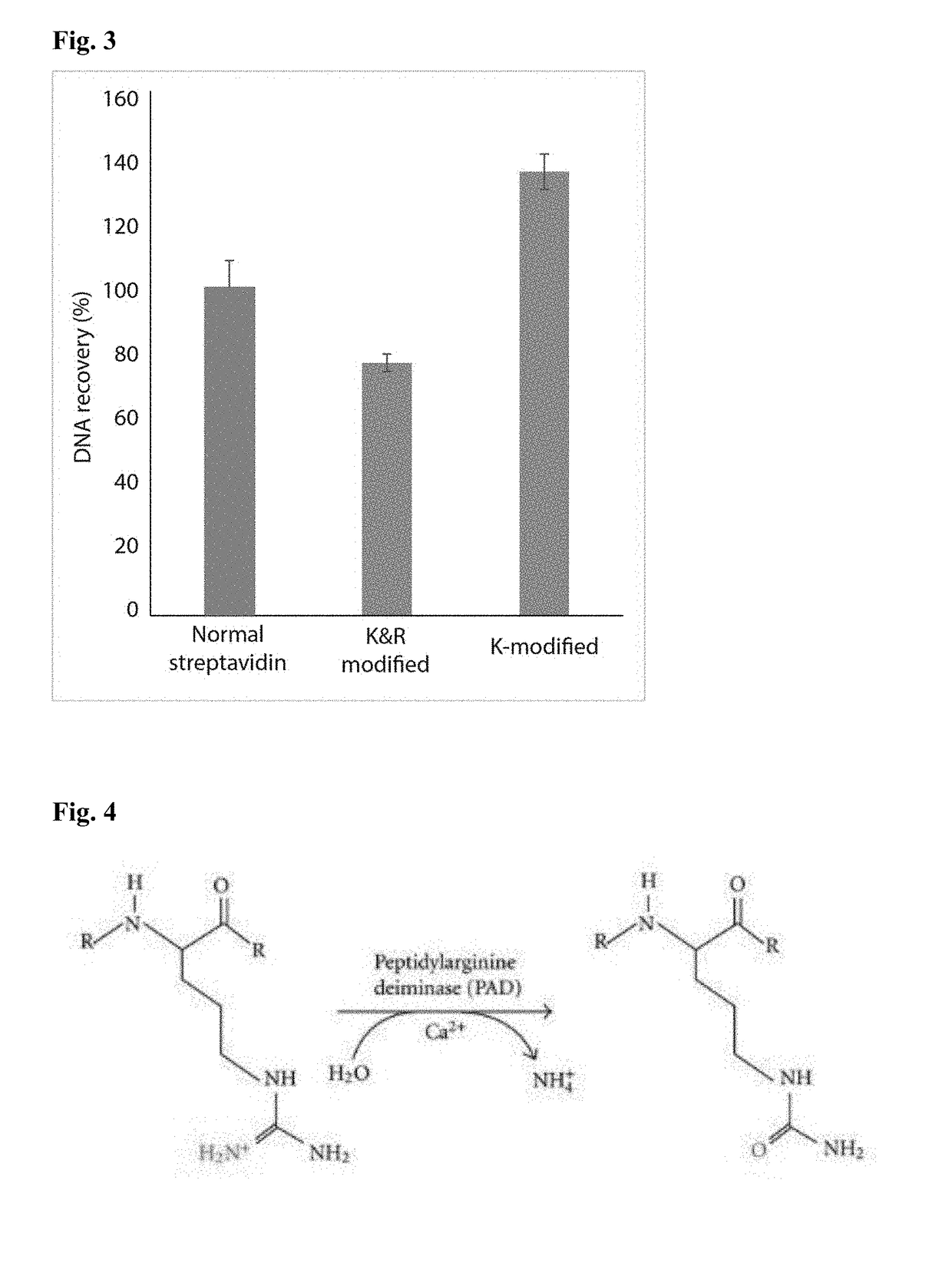
Protease-resistant streptavidin
The present invention relates to modified streptavidin molecules that are resistant to cleavage by Lys-C or other proteases. These modified streptavidin molecules can be produced by chemical modification of natural streptavidin, by chemical synthesis or by genetic engineering. The invention also relates to nucleic acid molecules encoding these modified streptavidin molecules, to vectors comprisingsuch nucleic acid molecules, and to cells comprising such nucleic acid molecules or vectors. The invention further relates to solid supports and kits comprising the modified streptavidin molecules. The invention also relates to the use of such modified streptavidin molecules or such solid supports for the capture / immobilization of proteins, peptides, oligonucleotides (e.g. aptamers), polynucleotides (e.g. DNA, RNA, or PNA), lipids, (poly) saccharides, carbohydrates, metabolites, drugs and small molecules, natural and synthetic molecules and to the use of these modified streptavidin moleculesor these solid supports in mass spectrometry for the identification of proteins that interact with aforementioned (bio)molecules. The invention further relates to a method for reducing background in mass spectrometry by employing the modified streptavidin molecules.
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL
Protease-resistant streptavidin
The present invention relates to modified streptavidin molecules that are resistant to cleavage by Lys-C or other proteases. These modified streptavidin molecules can be produced by chemical modification of natural streptavidin, by chemical synthesis or by genetic engineering. The invention also relates to nucleic acid molecules encoding these modified streptavidin molecules, to vectors comprising such nucleic acid molecules, and to cells comprising such nucleic acid molecules or vectors. The invention further relates to solid supports and kits comprising the modified streptavidin molecules. The invention also relates to the use of such modified streptavidin molecules or such solid supports for the capture / immobilization of proteins, peptides, oligonucleotides (e.g. aptamers), polynucleotides (e.g. DNA, RNA, or PNA), lipids, (poly) saccharides, carbohydrates, metabolites, drugs and small molecules, natural and synthetic molecules and to the use of these modified streptavidin molecules or these solid supports in mass spectrometry for the identification of proteins that interact with aforementioned (bio)molecules. The invention further relates to a method for reducing background in mass spectrometry by employing the modified streptavidin molecules.
Owner:EURO LAB FUER MOLEKULARBIOLOGIE EMBL
Models of prion disease
InactiveUS20020004938A1Increase infectivityCompound screeningFungiDisease progressionMechanical Processes
The present invention provides a novel PrP protein, and nucleic acids encoding this protein, where the PrP protein is characterized in vivo by 1) incomplete glycosylation relative to glycosylation of wild-type PrPC and 2) proper cellular localization, i.e. an ability to be transported to the cell surface. This novel, under-glycosylated PrP, unlike its normal cellular counterpart, can easily be converted into a protease-resistant isoform by incubation with infectious prions. The invention further provides systems for the study of prion disorders and methods of using these systems, e.g. the study of the mechanical processes in progression of prion-mediated disease or the identification of new therapeutic agents for treatment of prion-mediated disorders. In such systems, protease-resistant under-glycosylated PrP is generated de novo and can be detected by standard immunoblot techniques.
Owner:RGT UNIV OF CALIFORNIA
Proteolysis resistant antibody preparations
Antibody preparations with substantially homogeneous and unsialylated glycoforms, such as G0 and G2, are prepared by enzymatic treatment, expression under certain conditions, use of particular host cells, and contact with serum. These antibody preparations resist cleavage by proteases, such as papain, ficin, bromolein, pepsin, a matrix metalloproteinase, such as MMP-7, neutrophil elastase (HNE), stromelysin (MMP-3) and macrophage elastase (MMP-12), and glycosylation modification enzymes. The antibody preparations with substantially homogeneous and unsialylated glycoforms and methods of testing for glycosylation in an antibody are useful in connection with characterization of antibody properties and / or in diseases or conditions characterized by an increase in protease activity.
Owner:JANSSEN BIOTECH INC
Pegylated single-modified recombinant glutathione peroxidase, preparation method and application of pegylated single-modified recombinant glutathione peroxidase
PendingCN111893100AImproves pH toleranceImprove thermal stabilityCosmetic preparationsPeptide/protein ingredientsSuccinic acidDrug efficiency
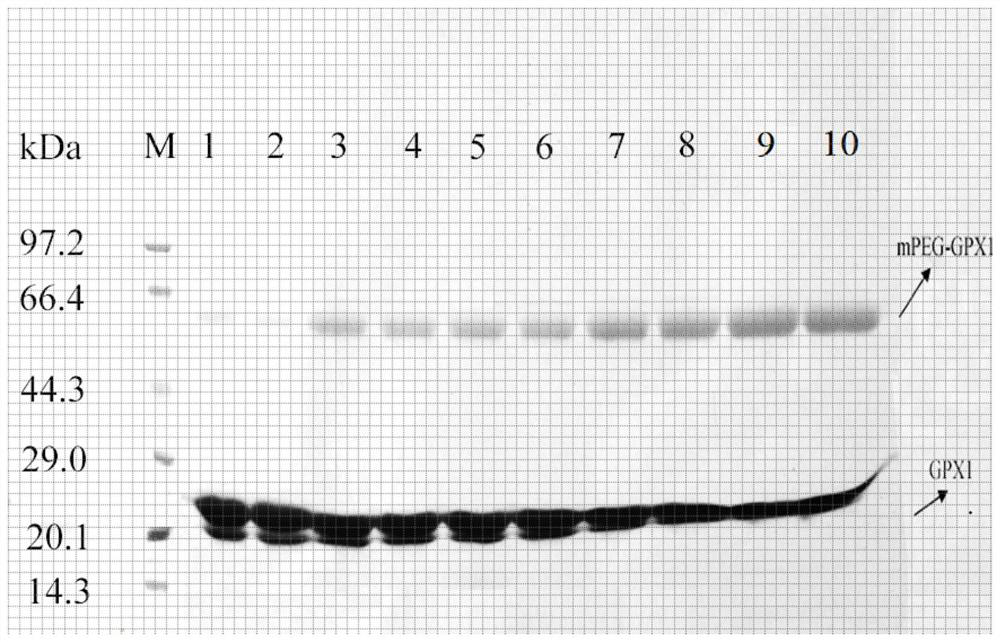
The invention discloses pegylated single-modified recombinant glutathione peroxidase (mPEG-GPX), and a preparation method and application of the pegylated single-modified recombinant glutathione peroxidase (mPEG-GPX) in preparation of antioxidant drugs, health care products and cosmetics, and belongs to the technical field of biology. The preparation method comprises the following step of carryingout covalent coupling on activated linear-chain monomethoxy polyethylene glycol succinimide succinate (mPEG-SS) and an amino group of recombinant glutathione peroxidase (GPX) to obtain mPEG-GPX. After GPX is modified by polyethylene glycol, the stability of the product mPEG-GPX is obviously enhanced compared with that of the GPX, the in-vivo antioxidant effect of mPEG-GPX is superior to that of the GPX, and development and utilization of mPEG-GPX health care products are facilitated; according to the obtained pegylated single-modified recombinant glutathione peroxidase, the pH tolerance, thethermal stability and the protease hydrolysis resistance of the recombinant glutathione peroxidase are improved, the circulating half-life period of the recombinant glutathione peroxidase in vivo is effectively prolonged, the stability of a drug is improved, and the drug effect is improved.
Owner:JILIN UNIV
Hinge region modification body of human IgG2 antibody
ActiveCN104177496AImprove hydrolysis effectImmunoglobulins against animals/humansSkeletal disorderHeavy chainHinge region
The invention discloses a hinge region modified recombinant human IgG2 antibody. Amino acids at the sites of Glu216Arg217Lys218 are deleted in a heavy chain hinge region of the antibody, and amino acid is substituted and / or deleted at the site of Cys219 and / or Cys220. The invention further provides a method for improving the antiprotease hydrolysis effect of the recombinant human IgG2 antibody. According to the IgG2 antibody provided by the invention, the heterogeneity caused by disulfide bonds in the hinge region of the antibody is eliminated, and the effect of improving the antiprotease hydrolysis effect is achieved.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Novel antiprotease acid alpha-galactosidase AGA36 and gene and application thereof
ActiveCN101712930AAppropriate pH valueStrong protease resistanceFungiBacteriaFood additiveBiotechnology
The invention relates to the field of gene engineering, in particular providing a novel antiprotease acid alpha-galactosidase AGA36 and a gene and an application thereof. The amino acid sequence of the alpha-galactosidase AGA36 is shown in SEQ ID NO.1, and the enzyme has appropriate acting pH value, strong resistance to metal ions and surfactants, strong protease resistance and better capacity for hydrolyzing various substrates, and can be applied to the feedstuff and food service industries as a feedstuff or a food additive.
Owner:GUANGDONG VTR BIO TECH
A high temperature high specific activity acidic β-mannanase and its gene and application
ActiveCN103525792BAcidicTaller than aliveFungiMicroorganism based processesBiotechnologyHeat resistance
The invention relates to the field of genetic engineering, specifically to high-temperature high-specific activity acidic beta-mannanase Man5A and a coding gene and application thereof. The amino acid sequence of the Man5A is represented by SEQ ID No. 1 or SEQ ID No. 2. The invention provides a novel mannanase gene; and mannanase coded by the gene has the advantages of acidity, high temperature, high specific activity, good heat resistance and good antiprotease capacity and can be used in the industries like feeds, food, medicines. With a technical scheme provided by the invention, production of mannanase with excellent properties and industrial applicability can be realized by using genetic engineering means.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
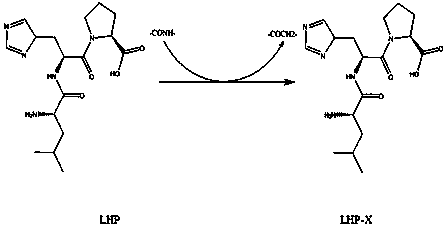
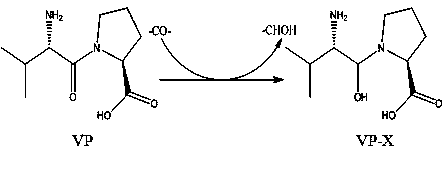
Method for improving structure of ACE inhibitory peptide prepared from food protein
InactiveCN104140456AMaintain inhibitory activityPromote degradationPeptidesOrganic synthesisDrug biological activity
The invention relates to a method for improving the structure of ACE inhibitory peptide prepared from food protein, and belongs to the biotechnology field. The method specifically includes the steps that the ACE inhibitory peptide prepared from the food protein is decomposed and identified through the technological means of ultra-filtration, gel chromatography, high performance liquid chromatography, mass spectrum analysis and the like, and an amino acid sequence of the ACE inhibitory peptide is clear and definite; then one to two amido bonds in the amino acid sequence of the ACE inhibitory peptide are replaced by groups such as -CHOH-, -COCH2- and -CH2CO- to form mimetic peptide substance of a non-peptide-bond structure; finally the mimetic peptide obtained after the structure improvement is synthesized with the organic synthesis method, and large-scale preparing is achieved. By means of the ACE inhibitory peptide improved with the method, the inhibitory activity can be well kept, the protease and peptidase degradation resisting capacity can be improved, and the biological activity can be improved.
Owner:GUANGDONG OCEAN UNIVERSITY
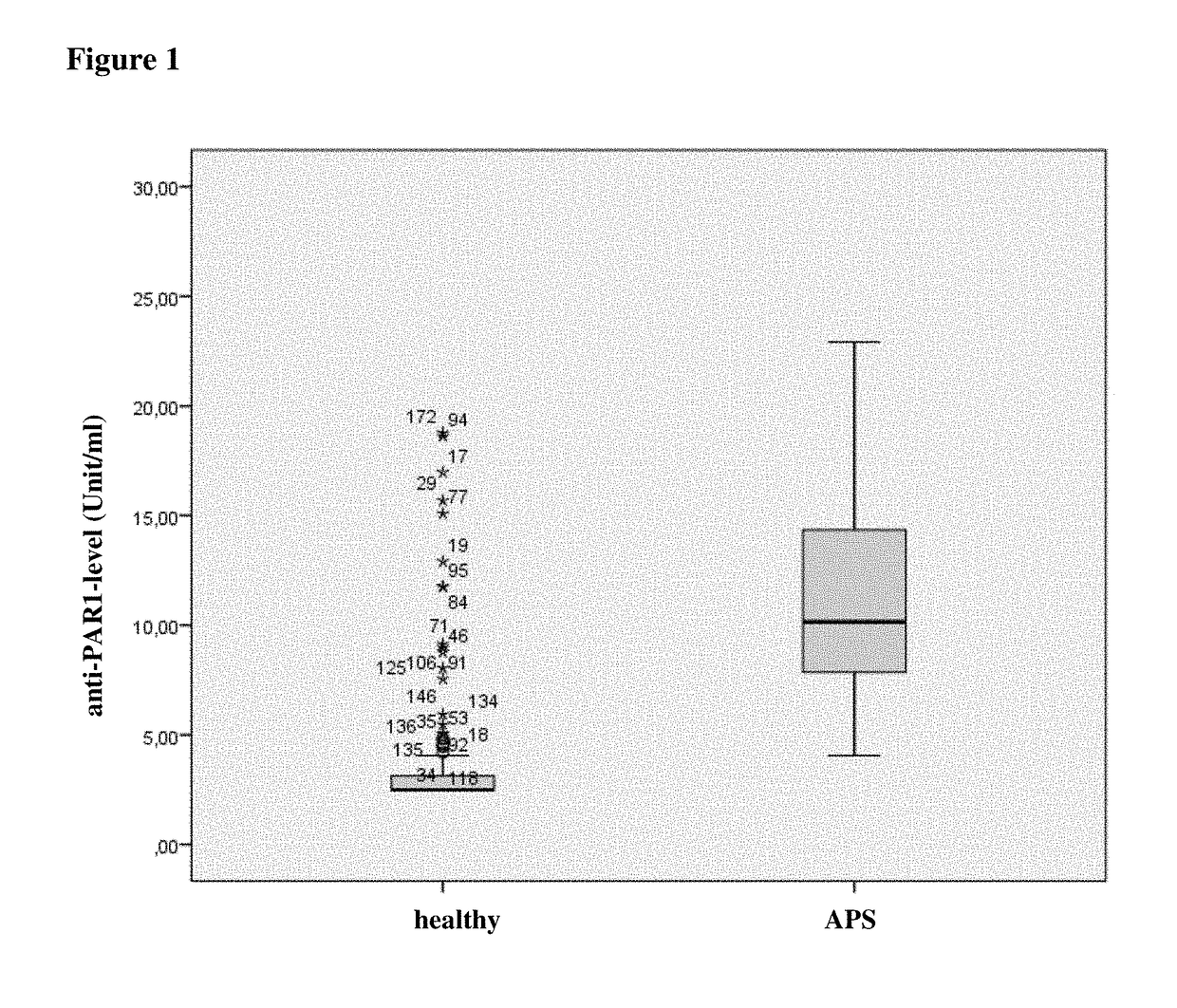
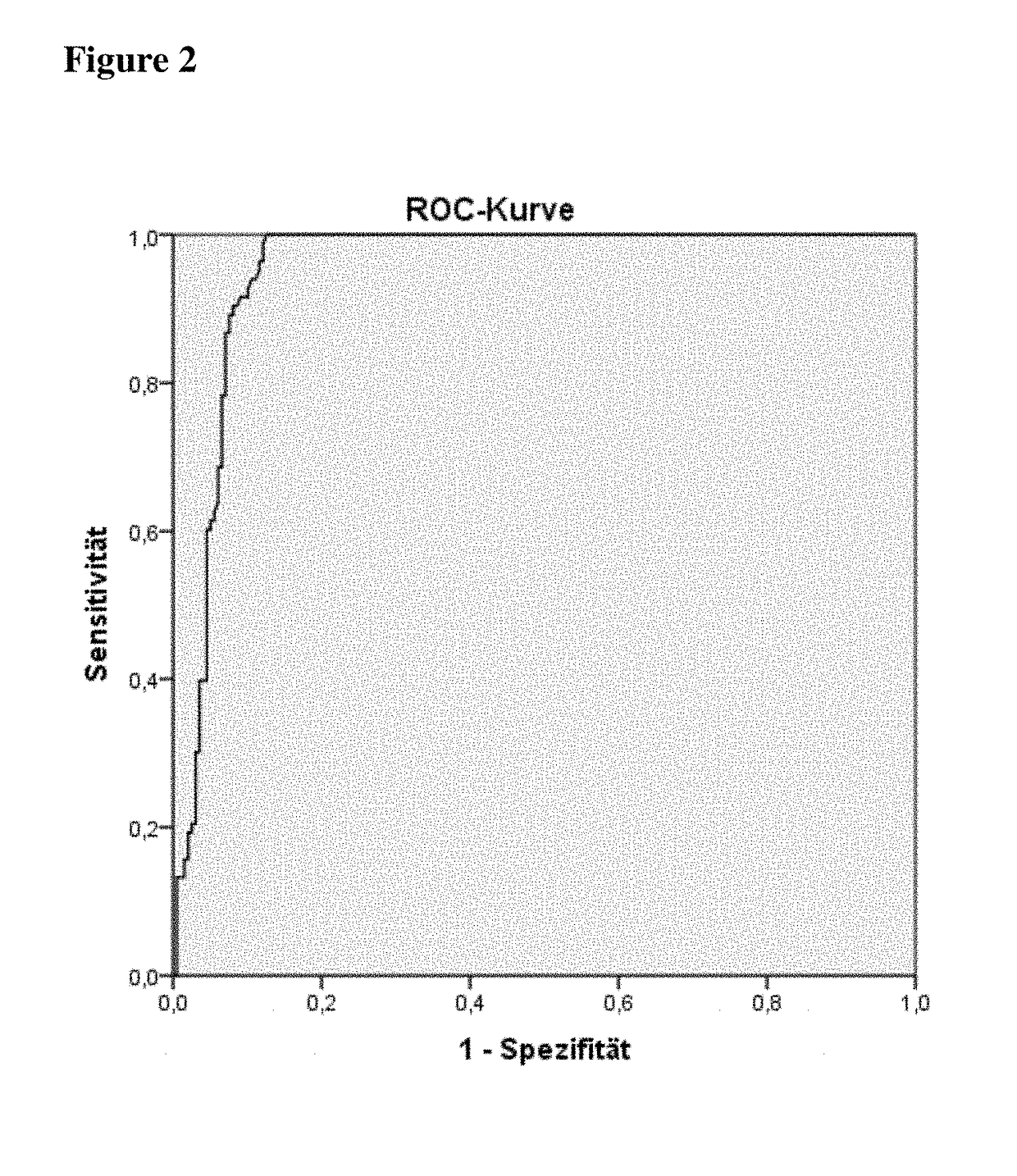
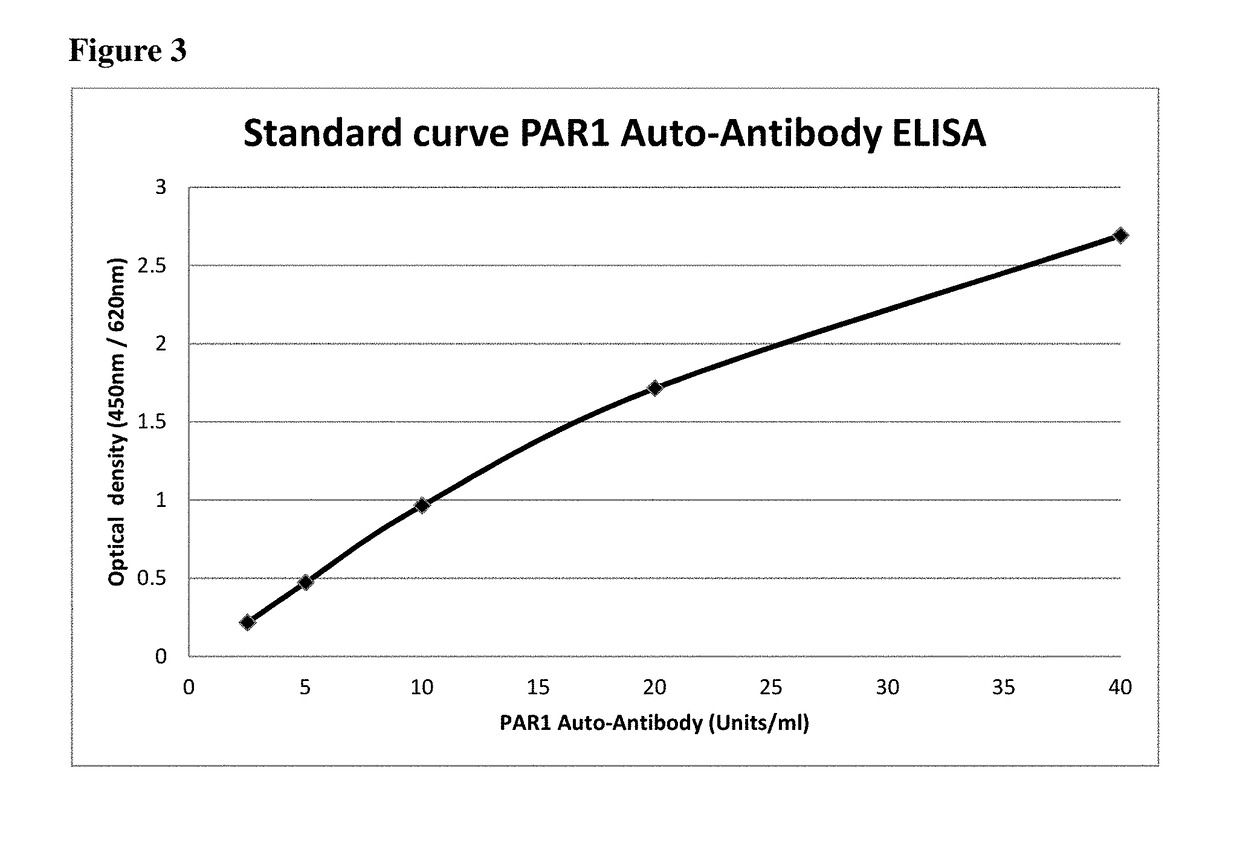
Method for diagnosing APS using determination of anti-PAR1 antibodies
The application relates to a method for diagnosis of an antiphospholipid syndrome (APS) in a subject, wherein presence or absence of an anti-protease-activated receptor 1 (PAR1) antibody is detected in a sample from the subject diagnosed, and wherein the presence of an anti-PAR1 antibody is indicative of the disease. Furthermore, it relates to the use of PAR1 for the diagnosis of APS, as well as to a method of removing anti-PAR1 antibodies from isolated blood of a subject upon detection of said anti-PAR1 antibodies in a sample of said patient.
Owner:CELLTREND
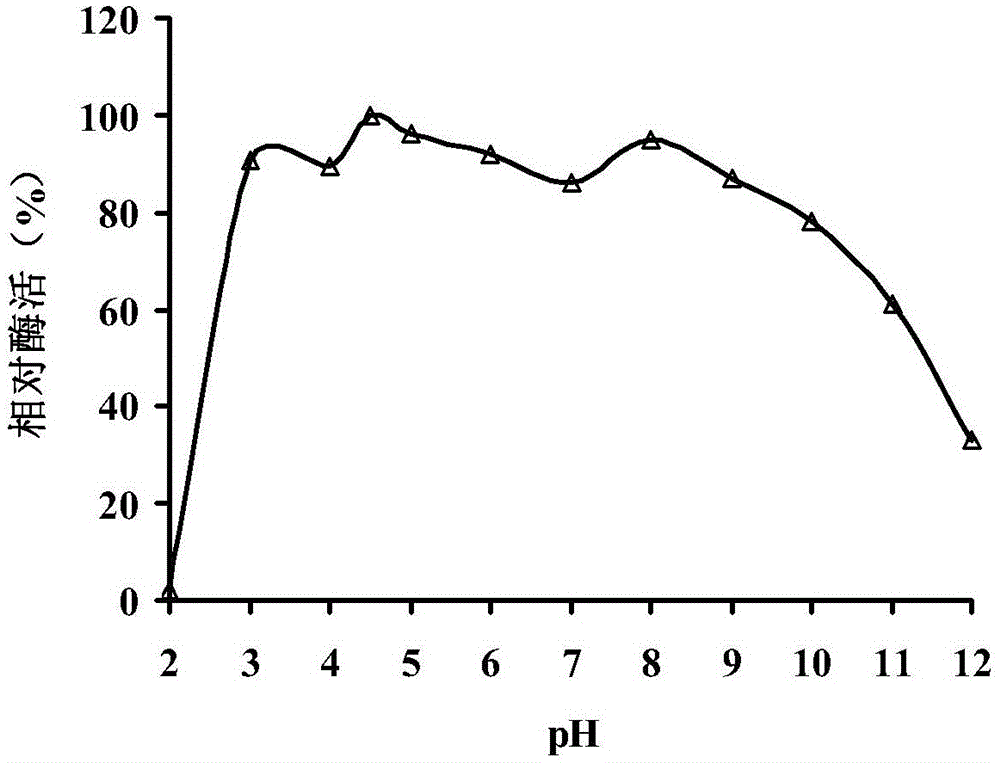
Beta-mannaseBA-Man5A with wide pH range, gene thereof and application of gene
The invention relates to the field of gene engineering, in particular to beta-mannaseBA-Man5A with a wide pH range, a gene thereof and application of the gene. The invention provides the beta-mannaseBA-Man5A with the wide pH range. The beta-mannaseBA-Man5A with the wide pH range has the amino acid sequence shown as SEQ ID NO.1 or 2. The invention also provides a gene for coding the beta-mannaseBA-Man5A with the wide pH range, a recombinant vector and a recombinant strain which comprise the gene, and application of the gene. The nucleotide sequence of the gene is shown as SEQ ID NO. 3 or 4. The beta-mannaseBA-Man5A has high activity in acidic, neutral and alkaline ranges, a wide pH range, high acid and alkali resistance, high heat resistance and excellent anti-protease ability, and can be applied to industries such as animal and fish feed, food, medicines, paper making and the like. According to the technical scheme of the invention, mannase can be produced by a gene engineering method.
Owner:SHANDONG LONGKETE ENZYME PREPARATION
Novel medicine use of lopinavir in alleviating toxicity of irinotecan
The present invention provides anti-HIV protease inhibitor lopinavir alone or the combination of lopinavir and ritonavir in the prevention or / and treatment of delayed diarrhea caused by an antineoplastic drug, diarrhea caused intestinal Use in drugs for dysfunction, wherein the antineoplastic drug refers to irinotecan or its active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). In the present invention, lopinavir has clear pharmacological effects, can effectively alleviate delayed diarrhea, and protect intestinal function.
Owner:BINZHOU MEDICAL COLLEGE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com