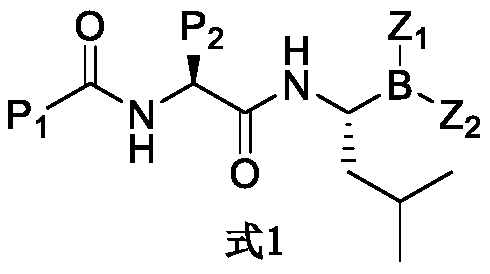
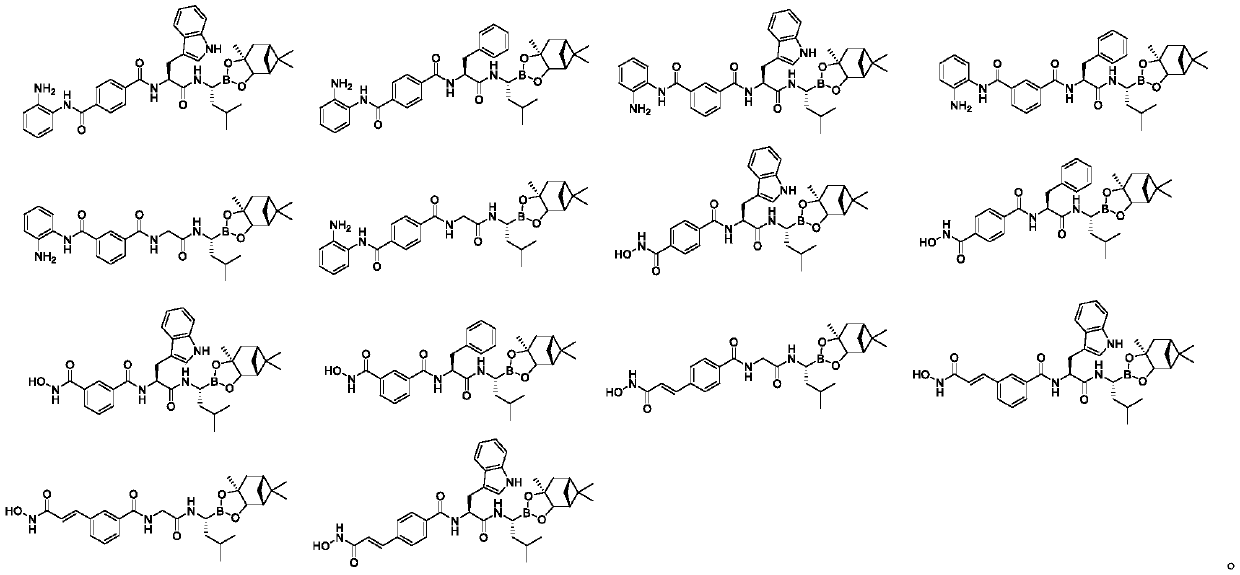
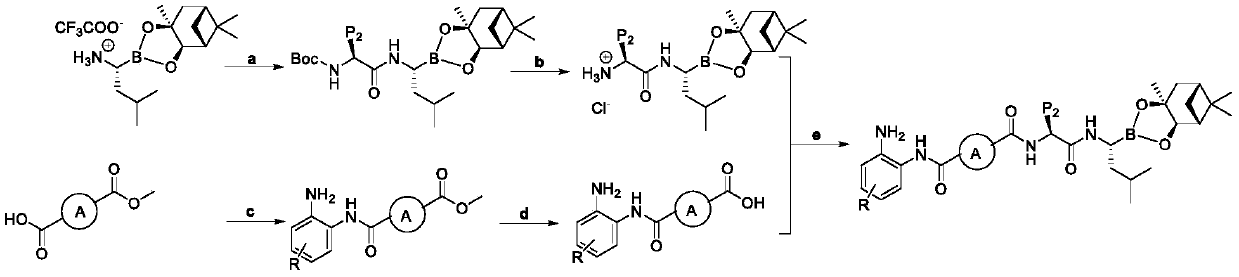
Histone deacetylase and proteasome double-target inhibitor, and preparation method and application thereof
A sirtuin and proteasome technology, applied in chemical instruments and methods, anti-inflammatory agents, drug combinations, etc., can solve problems such as poor patient compliance, pharmacokinetic mismatch, and drug interactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1: tert-butyl ((S)-1-((((R)-3-methyl-1-((3aR,4R,6R,7aS)-5,5,7a-trimethylhexahydro -4,6-Methylbenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropan-2-yl) Urethane (2a)
[0073] Boc-L-Phe (3.6g, 13mmol) was dissolved in N,N-dimethylformamide under ice-cooling, and TBTU (4.2g, 13mmol), bortezomib intermediate I (4.56g, 12mmol) and N - Methylmorpholine (4ml, 36mmol). Under nitrogen protection, the reaction was continuously stirred for 16 hours, poured into water, and extracted 3 times with ethyl acetate (3×100 mL). The combined organic phases were washed with 2% citric acid, 2% sodium bicarbonate, and saturated brine, dried over magnesium sulfate, and spin-dried to obtain a crude product. Chromatography on silica gel eluting with ethyl acetate, petroleum ether gradient (from 15% to 40%) afforded 6 g (97%) of 2a as a colorless transparent oil. Yield: 95%. 1 HNMR (400MHz, DMSO-d 6 ):δ8.85(brs,2H),7.35–7.07(m,5H),7.03(d,J=8.3Hz,1H),4.28–4.18(m,1H),4.08...
Embodiment 2
[0078] Example 2: (S)-1-((((R)-3-methyl-1-((3aR,4R,6R,7aS)-5,5,7a-trimethylhexahydro-4,6 -Methoxybenzo[d][1,3,2]dioxaborol-2-yl)butyl)amino)-1-oxo-3-phenylpropane-2-ammonium chloride (3a )
[0079] 2a was dissolved in ethyl acetate saturated with hydrogen chloride and stirred overnight to obtain pure white powder 3a, yield: 90%, melting point: 208-212°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.55(s,1H),8.29(s,3H),7.38–7.20(m,5H),4.29(dd,J=7.7,2.2Hz,1H),3.99(s,1H),3.00(d ,J=8.1Hz,2H),2.88(d,J=5.0Hz,1H),2.35–2.24(m,1H),2.12(dd,J=10.5,5.8Hz,1H),1.94(t,J= 5.5Hz, 1H), 1.85(s, 1H), 1.71(dt, J=14.3, 2.7Hz, 1H), 1.47(t, J=6.5Hz, 1H), 1.36–1.15(m, 9H), 0.81– 0.80(m,9H).
[0080] 2-((((R)-3-methyl-1-((3aR,4R,6R,7aS)-5,5,7a-trimethylhexahydro-4,6-methoxybenzo[d ][1,3,2]dioxaborol-2-yl)butyl)amino)-2-oxa-1-amine ammonium chloride (3b)
[0081] Similar to the synthesis of 3a, yield: 98%, colorless oil. 1 H NMR (400MHz, DMSO-d 6)δ8.48(d,J=5.2Hz,1H),8.10(s,3H),4.29(d,J=7.2Hz,1H),3.54(s,2H),3....
Embodiment 3
[0084] Example 3: Methyl 4-((2-aminophenyl)carbamoyl)benzoate (5a)
[0085] Monomethyl terephthalate (4a, 1.8g, 10mmol), o-phenylenediamine (1.08g, 10mmol), TBTU (3.5g, 12mmol) were dissolved in N,N-dimethylformamide under ice cooling. Join Et 3 N (1.5ml, 30mmol) was stirred for 8 hours in the dark. After the reaction was completed, it was poured into water and extracted three times with ethyl acetate (3×100 mL). The combined organic phases were washed with 2% citric acid, 2% sodium bicarbonate, and saturated brine, dried over magnesium sulfate, and spin-dried to obtain a crude product. The refined product 5a was obtained after methanol recrystallization. Yield: 56%, melting point: 190-194°C. 1 H NMR (400MHz, CDCl 3 )δ8.16(d, J=7.9Hz, 2H), 7.99(dd, J=15.1, 8.3Hz, 2H), 7.37(d, J=7.9Hz, 1H), 7.12(dd, J=8.7, 5.3 Hz,1H),6.87(t,J=6.3Hz,2H),3.97(s,3H),3.86(s,1H).
[0086] Methyl 3-((2-aminophenyl)carbamoyl)benzoate (5b)
[0087] Similar to the synthesis of 3a, yield: 56%, ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com