Novel antiprotease acid alpha-galactosidase AGA36 and gene and application thereof
A galactosidase and protease-resistant technology, applied in the field of new α-galactosidase AGA36 and its gene, and the recombinant vector containing the gene, can solve the problem of inability to hydrolyze various substrates, and achieve excellent hydrolysis of various Substrate capacity, effect of strong protease resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
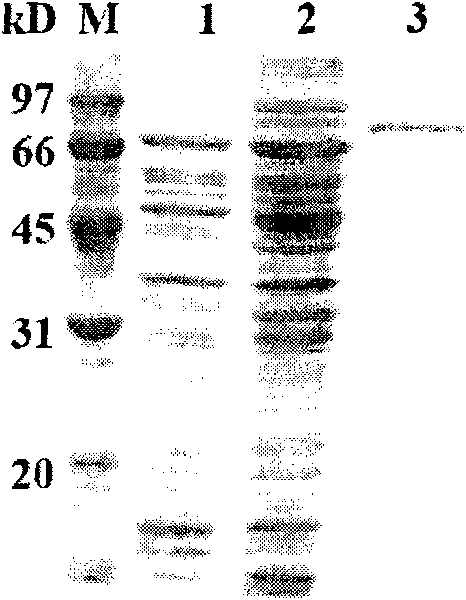
Image
Examples
Embodiment 1
[0042] Example 1 Isolation of Rhizopus AG708 (Rhizopus.sp.AG708) and its enzyme-producing properties
[0043] After the strain Rhizopus AG708 (Rhizopus.sp.AG708) was grown on PDA medium for 3-5 days, the primers designed according to the conserved sequence of filamentous fungal 18S rDNA were used to amplify the 18S rDNA of the strain by PCR, and the sequencing results were compared with those in the Genbank database. According to the nucleotide sequence comparison of AG708, the 18s rDNA nucleotide sequence of AG708 has the highest similarity of 99% with Rhizopus.oryzae CBS 278.38 (Genbank accession No.AB250174), and with R. ) and R.caespitosus CBS427.87 (Genbank accession No.AB250168) have a similarity of 97%, which proves that the bacterial strain is Rhizopus, and it is named Rhizopus.sp.AG708. Soybean meal was added as an inducer and carbon source to the enzyme-producing medium (4% K 2 HPO 4 , 0.28% (NH 4 ) 2 SO 4 , 0.12% CaCl 2 , 0.12% Urea, 0.12% MgSO 4 , 0.02% Mann...
Embodiment 2
[0044] Cloning of embodiment 2 Rhizopus α-galactosidase coding gene aga36
[0045] Genomic DNA extraction of Rhizopus (Rhizopus.sp.AG708): take the Rhizopus liquid cultured at 30°C for 7 days and centrifuge at 6000rpm for 10min. Take 100mg of mycelia and add 500μL of sterile water to wash, centrifuge to get the precipitate. The precipitate was resuspended in 500 μL extract mixture, incubated at 37°C for 60 min, and centrifuged at 10,000 rpm for 10 min to remove the precipitate. The supernatant was extracted sequentially with equal volumes of phenol, phenol:chloroform, and chloroform. Take the upper layer solution and add 0.6-1 times the volume of isopropanol to precipitate at room temperature for 10 minutes. Centrifuge at 12000rpm for 15min. The precipitate was washed with 70% ethanol, centrifuged slightly, dried and dissolved in 30 μL sterile water for later use.
[0046] The degenerate primers P1: 5'-TYGTBMTKGAYGAYGGYTGG-3' and P2: 5'-GACCATYTCNGGYTCNAMCC-3' were designe...
Embodiment 3
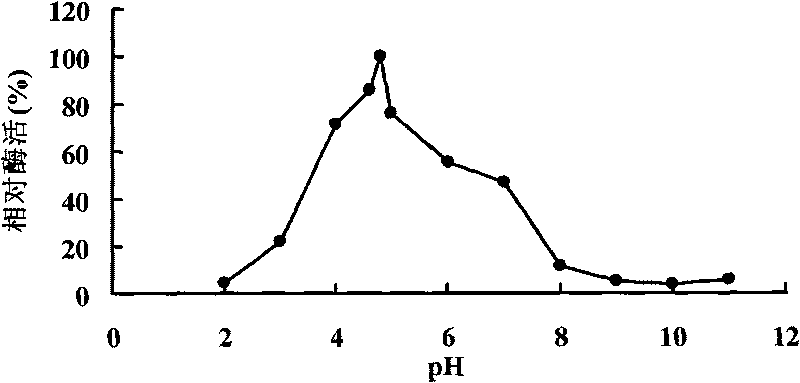
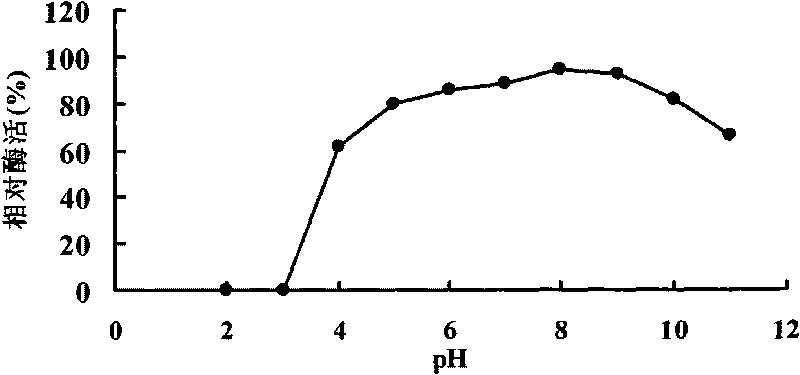
[0048] Example 3 Activity analysis of α-galactosidase.
[0049] Enzyme activity was determined by the pNPG method. Dissolve pNPG in 0.1mol / L McIlvaine buffer to make the final concentration 2mmol / L. Mix 20 μL of enzyme solution, 230 μL of McIlvaine buffer and 250 μL of 2mM pNPG, and shake well. After incubating at 37°C for 5 min, 1.5 mL of 1M Na was added to the reaction solution 2 CO 3 solution to terminate the reaction. The OD value was measured at 405nm, and the enzyme activity was expressed by the production of p-nitrophenol (pNP). After adding enzyme solution and buffer to the control tube, add Na first 2 CO 3 The solution was then added with pNPG solution.
[0050] Enzyme activity (U / mL) unit definition: The amount of enzyme needed to decompose pNPG to release 1 μmol pNP per minute at 37°C is defined as one enzyme activity unit.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com