Pegylated single-modified recombinant glutathione peroxidase, preparation method and application of pegylated single-modified recombinant glutathione peroxidase
A technology of glutathione peroxidase and glutathione peroxidase, which is used in polyethylene glycol modification of protein compounds, preparation of antioxidant drugs, applications in health care products and cosmetics, preparation, polyethylene glycol The field of alcoholated single-modified recombinant glutathione peroxidase can solve the problems of increased separation and purification challenges and costs, and achieve the effects of prolonging circulation half-life, improving pH tolerance and thermal stability, and resisting protease hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
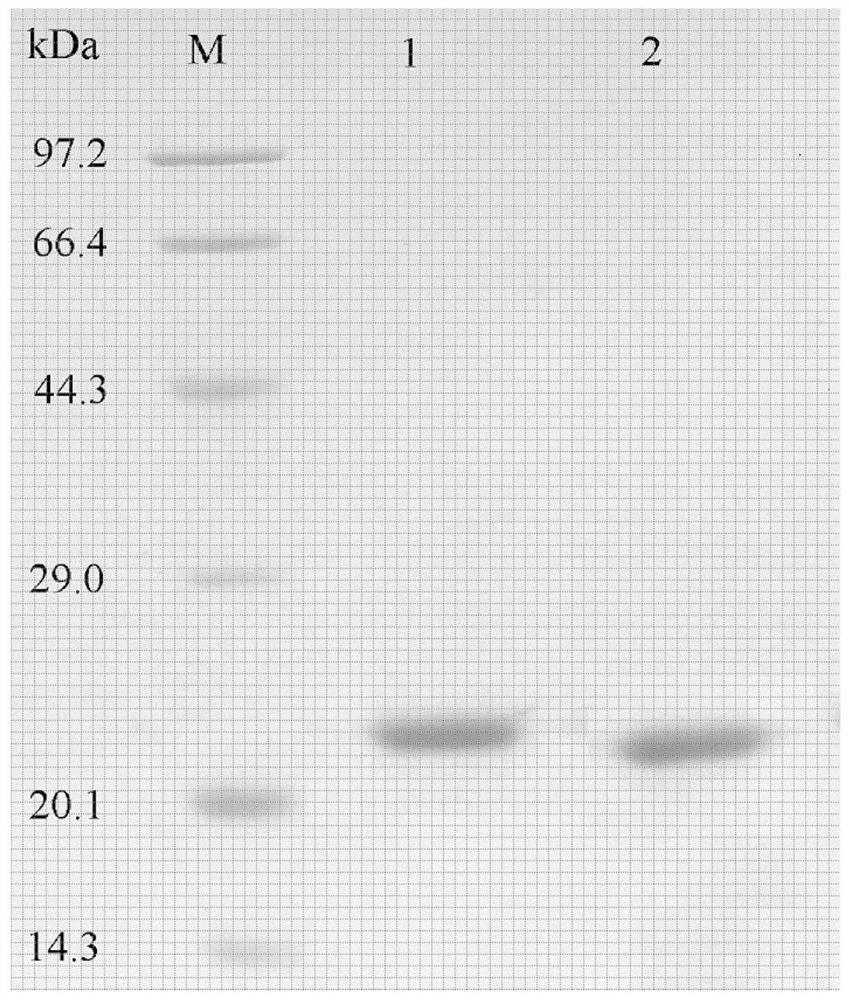
[0084] Example 1: PEGylated Single Modification of Recombinant Glutathione Peroxidase 1
[0085] (1) Acquisition of recombinant glutathione peroxidase 1
[0086] Recombinant glutathione peroxidase 1 (GPX1) was prepared according to the method published in Chinese patent 201510431360.1. GPX1 target proteins were identified by SDS-PAGE and Western blot.
[0087] (2) Modification reaction
[0088] The activated linear monomethoxy polyethylene glycol succinimide succinate with an average molecular weight of 20kD is used as a modifier to covalently couple with the recombinant glutathione peroxidase GPX1 obtained in step (1) . In the reaction, the molar ratio of GPX1 to modifier was 1:30, and the covalent coupling reaction was carried out at 4°C and pH 8.5 for 30 minutes, and linear monomethoxypolyethylene glycol succinimide succinic acid was added The ester molar ratio is 1:1 and the terminator glycine terminates the reaction to obtain a linear monomethoxypolyethylene glycol su...
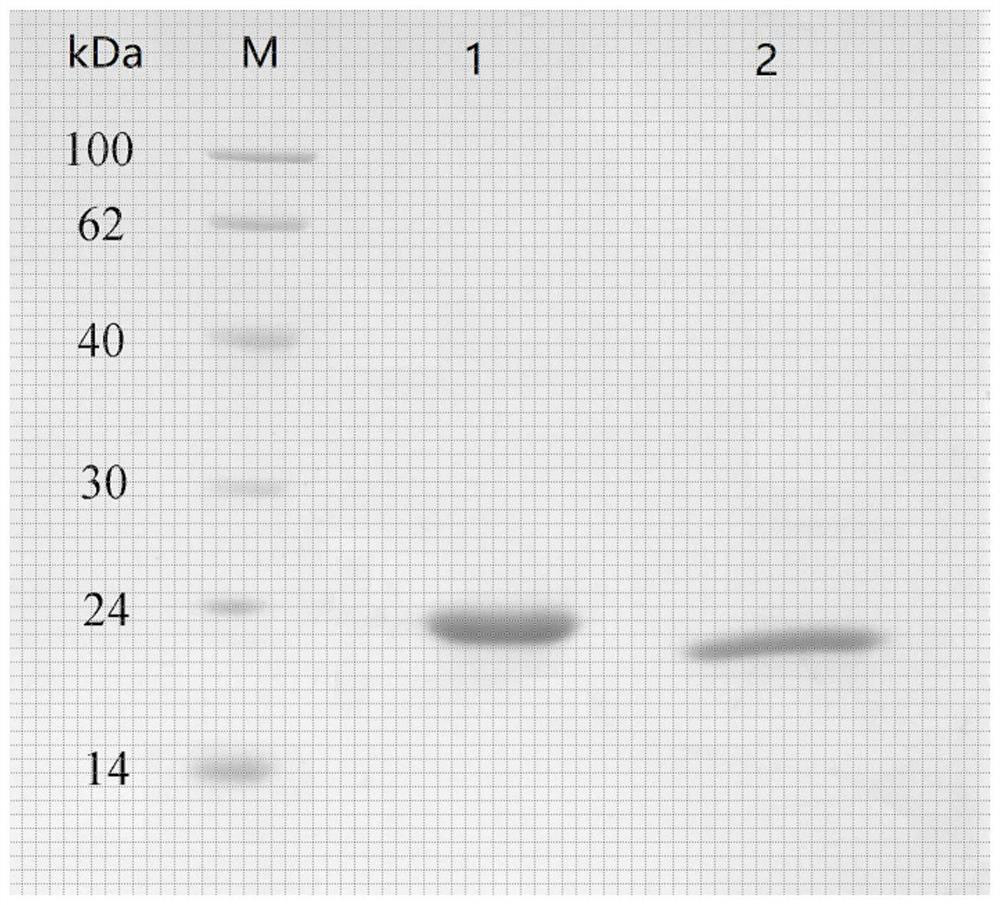
Embodiment 2
[0092] Example 2: PEGylated Single Modification of Recombinant Glutathione Peroxidase 4
[0093] (1) Acquisition of recombinant glutathione peroxidase 4
[0094] Recombinant glutathione peroxidase 4 (GPX4) was prepared according to the method published in Chinese patent 201510431360.1. First use the gene synthesis method to obtain the hybrid tRNA gene, then synthesize or amplify the GPX4 gene, and assemble the artificially synthesized hybrid tRNA and the gene sequences corresponding to its upstream and downstream promoters and terminators into the polyclonal secretory prokaryotic expression vector site, and then assemble the GPX4 gene into the multiple cloning site of the secretory prokaryotic expression vector, and use the genetic mutation method to mutate the coding sequence of selenocysteine in the active center of GPX4 to TAG, and transform UAG Read through the engineering bacteria, use hybrid tRNA to encode the UAG stop codon as selenocysteine in the nutrient agar me...
Embodiment 3
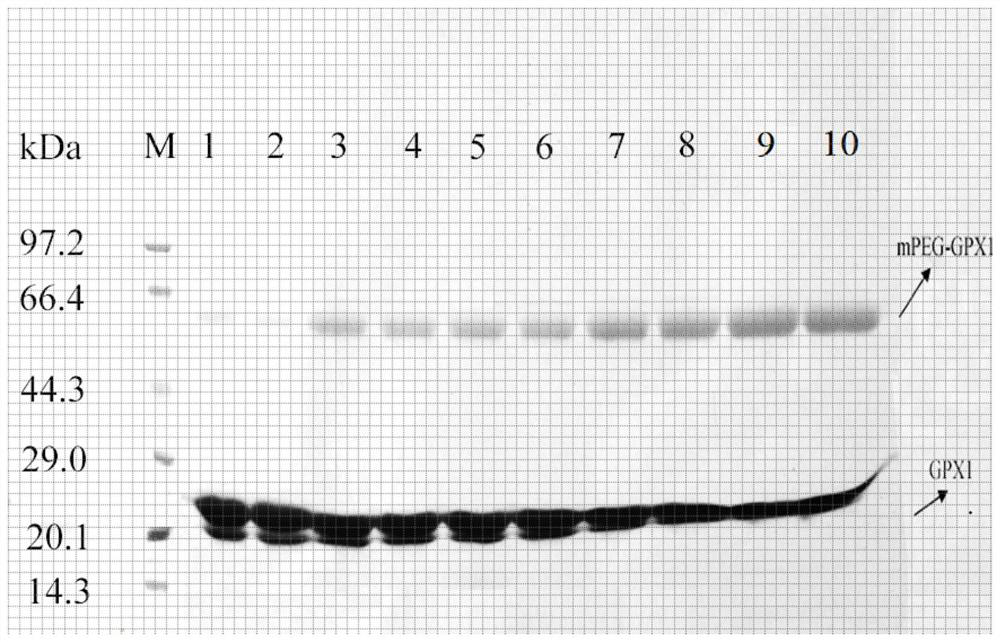
[0099] Embodiment 3: the physicochemical property of mPEG-GPX
[0100] (1) Circular Dichroism Spectrum
[0101] Circular dichroism analysis was performed using a circular dichroism spectrometer, and the secondary structures of GPX and mPEG-GPX were compared. The GPX samples (GPX1 and mPEG-GPX1, GPX4 and mPEG-GPX4) before and after polyethylene glycol modification were placed in 20mmol / L phosphate buffer at pH 8.5, and measured by BCA method and using 20mmol / L of pH 8.5 L phosphate buffer adjusted the final concentration of each sample to 0.1 mg / mL. Add the sample to a circular dichroic cuvette with a light path of 0.1 cm, place it in a J-810 circular dichroic spectrometer, and set it at room temperature and N 2 measured in the environment. The buffer spectrum served as a blank control and was removed from the spectrum of the protein solution. The wavelength scanning range is 195~250nm. Measure three times and take the average value.
[0102] Depend on Figure 7 It can b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com