Uricase/catalase lipid nanoparticles wrapped by fusion cell membrane and preparation method of uricase/catalase lipid nanoparticles
A technology of catalase lipid and catalase, which is applied in the direction of nanotechnology, nanotechnology, nanomedicine, etc., can solve the problems of low targeting, low bioavailability, poor stability, etc., and achieve good stability , good immunogenicity and enhanced stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
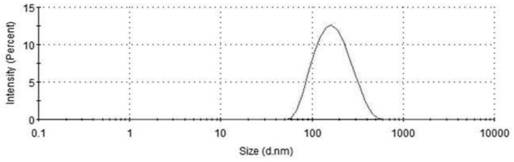
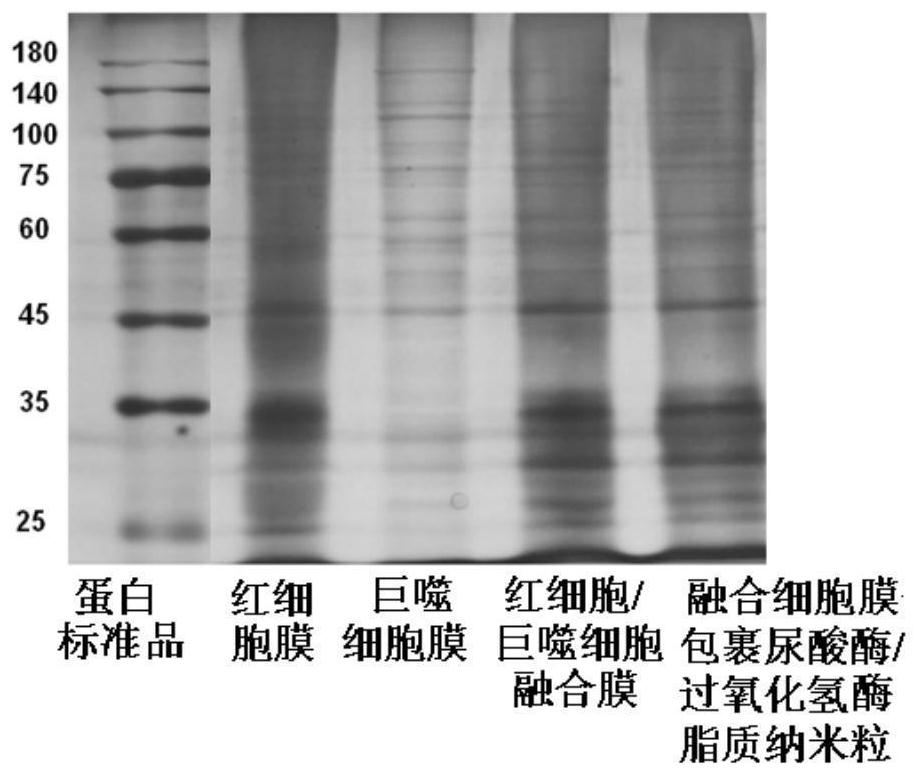
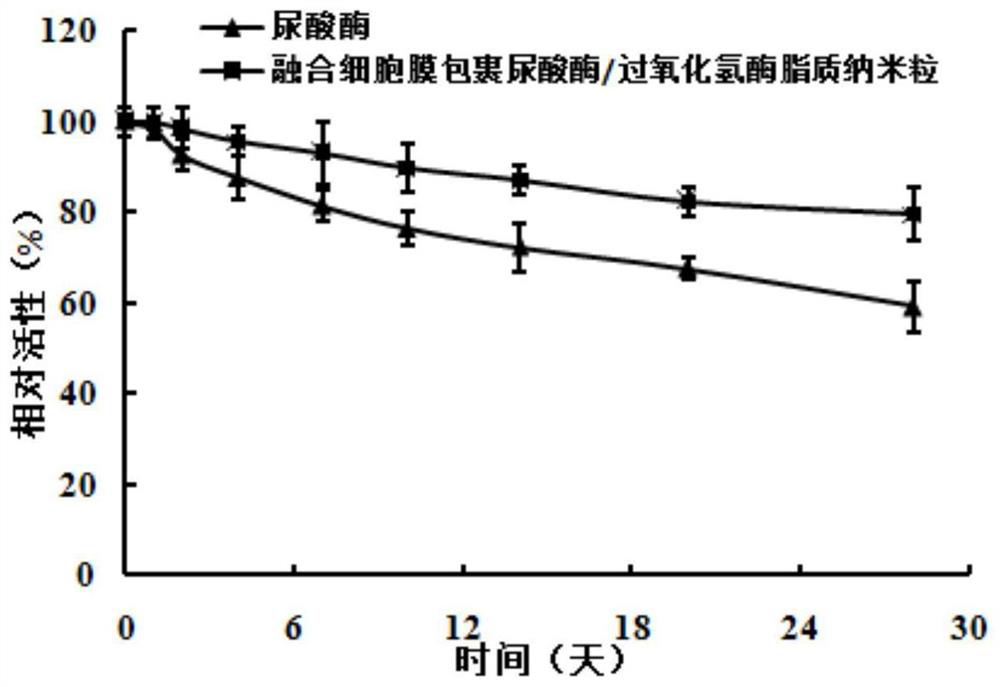
[0028] Fused cell membrane-wrapped uricase / catalase lipid nanoparticles include uricase / catalase lipid nanoparticles and erythrocyte / macrophage fusion cell membrane envelope. The weight ratio of each component in the uricase / catalase lipid nanoparticle formula is: 2 parts of lecithin, 1 part of cholesterol, 200 parts of boric acid-borax buffer solution, the buffer solution concentration is 50mM, and the buffer solution pH is 7, The uricase content was 0.1 mg / mL, and the catalase content was 0.05 mg / mL.
[0029] Preparation method: (1) Preparation method of uricase / catalase lipid nanoparticles: dissolve uricase and catalase in a buffer solution with a prescription amount in a mass ratio (2:1) to form uricase / catalase Catalase solution, weigh the prescribed amount of lecithin and cholesterol and dissolve in 10mL of dichloromethane organic solvent, remove the organic solvent by rotary evaporation to form a uniform lipid film, add uricase / catalase solution, shake in a water bath a...
Embodiment 2
[0031] Fused cell membrane-wrapped uricase / catalase lipid nanoparticles include uricase / catalase lipid nanoparticles and erythrocyte / macrophage fusion cell membrane envelope. The weight ratio of each component in the uricase / catalase lipid nanoparticle formula is: 2.7 parts of lecithin, 2.1 parts of cholesterol, 418.2 parts of N, N-bishydroxyethylglycine-sodium hydroxide buffer solution, buffer solution The concentration is 120mM, the pH of the buffer solution is 7.3, the content of uricase is 0.07mg / mL, and the content of catalase is 0.14mg / mL.
[0032] Preparation method: (1) Preparation method of uricase / catalase lipid nanoparticles: dissolve uricase and catalase in a buffer solution with a prescription amount in a mass ratio (1:2) to form uricase / catalase Catalase solution, weigh the prescribed amount of lecithin and cholesterol and dissolve in 15mL chloroform organic solvent, remove the organic solvent by rotary evaporation to form a uniform lipid film, add uricase / catala...
Embodiment 3
[0034] Fused cell membrane-wrapped uricase / catalase lipid nanoparticles include uricase / catalase lipid nanoparticles and erythrocyte / macrophage fusion cell membrane envelope. The weight ratio of each component in the uricase / catalase lipid nanoparticle formula is: 3.4 parts of lecithin, 2.6 parts of cholesterol, 527.3 parts of sodium carbonate-sodium bicarbonate buffer solution, buffer solution concentration is 130mM, buffer solution pH was 7.6, the uricase content was 0.1 mg / mL, and the catalase content was 0.15 mg / mL.
[0035] Preparation method: (1) Preparation method of uricase / catalase lipid nanoparticles: dissolve uricase and catalase in the buffer solution of prescription amount with mass ratio (1:1.5) to form uricase / catalase Catalase solution, weigh the prescribed amount of lecithin and cholesterol and dissolve in 20mL of anhydrous ethanol organic solvent, remove the organic solvent by rotary evaporation to form a uniform lipid film, add uricase / catalase solution, sha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com