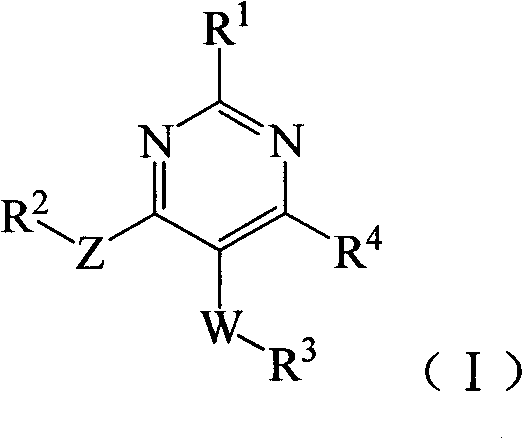
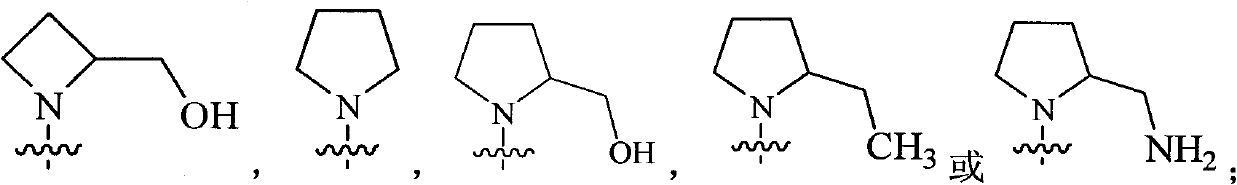
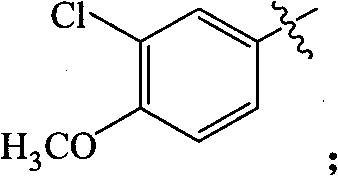
Substituted miazines compound
A technology of compounds and substituents, applied in the field of medicine, can solve problems such as life-threatening, long half-life, and low blood pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] The preparation of embodiment 1 3-chloro-4-methoxybenzylamine
[0177]
[0178] (1) Preparation of 3-chloro-4-methoxybenzyl alcohol
[0179]
[0180] Dissolve methyl 3-chloro-4-methoxybenzoate (1 g, 5 mmol) in 30 mL of dry THF, and slowly add LiAlH at -10 to -20 °C 4 (0.38g, 10mmol), monitored by TLC, after 2 hours of reaction, quenched with 4-5% NaOH solution, added 500mL ethyl acetate, there was a milky precipitate, suction filtered, and rotary evaporated to obtain 3-chloro-4-methoxy 0.78 g of benzyl alcohol, yield 91%.
[0181] (2) Preparation of 4-azidomethyl-2-chloromethoxybenzene
[0182]
[0183] 3-Chloro-4-methoxybenzyl alcohol (200mg, 1.16mmol) was dissolved in 20mL of dry THF, and DBU (210mg, 1.28mmol) and DPPA (320mg, 1.16mmol) were added slowly at 0°C, TLC After about 3 hours of reaction, stop the reaction, add an appropriate amount of saturated NaCl solution, extract with ethyl acetate, rotary evaporate, and pass through the column to obtain 1...
Embodiment 2
[0187] Example 2 (S)-4-(3-chloro-4-methoxybenzylamino)-2-[2-(hydroxymethyl)tetrahydropyrrol-1-yl]-6-methyl-N- Preparation of (pyrimidine-2-methyl)pyrimidine-5-acetamide (compound 1)
[0188]
[0189] (1) Preparation of ethyl 4-(3-chloro-4-methoxybenzylamino)-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylate
[0190]
[0191] Dissolve ethyl 4-chloro-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylate (700mg, 2.8mmol), triethylamine (2mL) in 50mL of anhydrous tetrahydrofuran, add slowly under ice-cooling 3-Chloro-4-methoxybenzylamine (486mg, 2.8mmol), stirred at room temperature for 12 hours, concentrated the reaction solution, added ethyl acetate for extraction, concentrated to give brown solid 4-(3-chloro-4-methoxy Benzylamino)-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylic acid ethyl ester 970 mg, yield 91%.
[0192] (2) Preparation of 4-(3-chloro-4-methoxybenzylamino)-6-methyl-2-(methylmercapto)pyrimidine-5-carboxylic acid
[0193]
[0194] Dissolve ethyl 4...
Embodiment 3
[0206] Example 3 (S)-4-(3-chloro-4-methoxybenzamide)-2-[2-(hydroxymethyl)tetrahydropyrrol-1-yl]-N-(pyrimidine-2- Preparation of methyl) pyrimidine-5-acetamide (compound 2)
[0207]
[0208] (1) Preparation of 4-(3-chloro-4-methoxybenzamide)-2-(methylmercapto)pyrimidine-5-carboxylic acid
[0209]
[0210] 3-Chloro-4-methoxybenzoyl chloride (1.86g, 9mmol) was dissolved in pyridine, and 4-amino-2-(methylmercapto)pyrimidine-5-carboxylic acid (1.67g, 9mmol) was weighed and added to pyridine solution, stirred and heated at 90°C, reacted for 18 hours, returned to room temperature, poured into dilute hydrochloric acid, extracted with dichloromethane, dried over anhydrous sodium sulfate, and spin-dried until a precipitate was precipitated, and the off-white solid 4-(3-chloro -4-methoxybenzamide)-2-(methylmercapto)pyrimidine-5-carboxylic acid 200 mg, yield 6.3%.
[0211] (2) Preparation of 4-(3-chloro-4-methoxybenzamide)-2-(methylmercapto)-N-(pyrimidine-2-methyl)pyrimidine-5-a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com