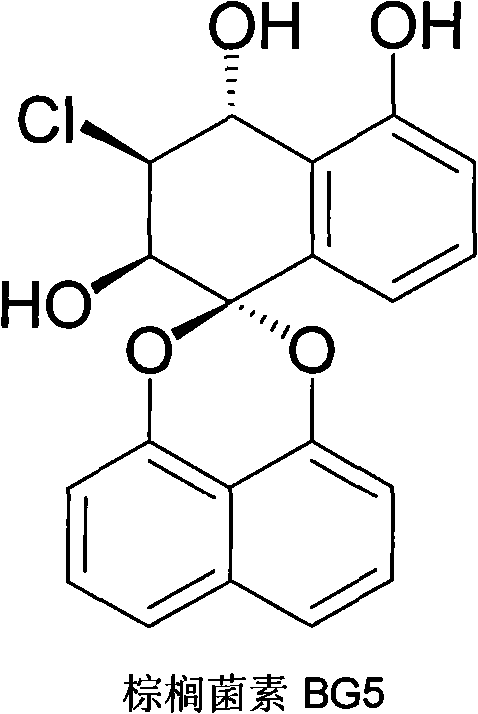
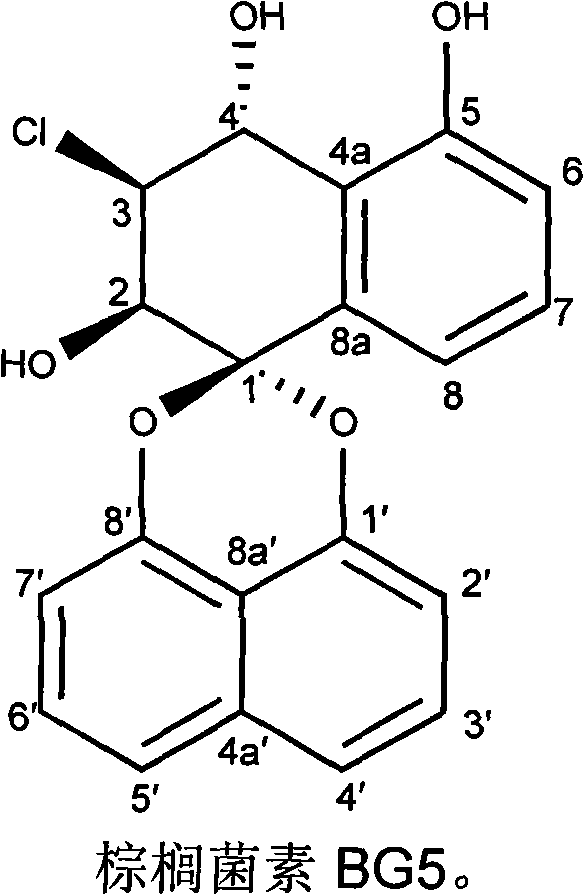
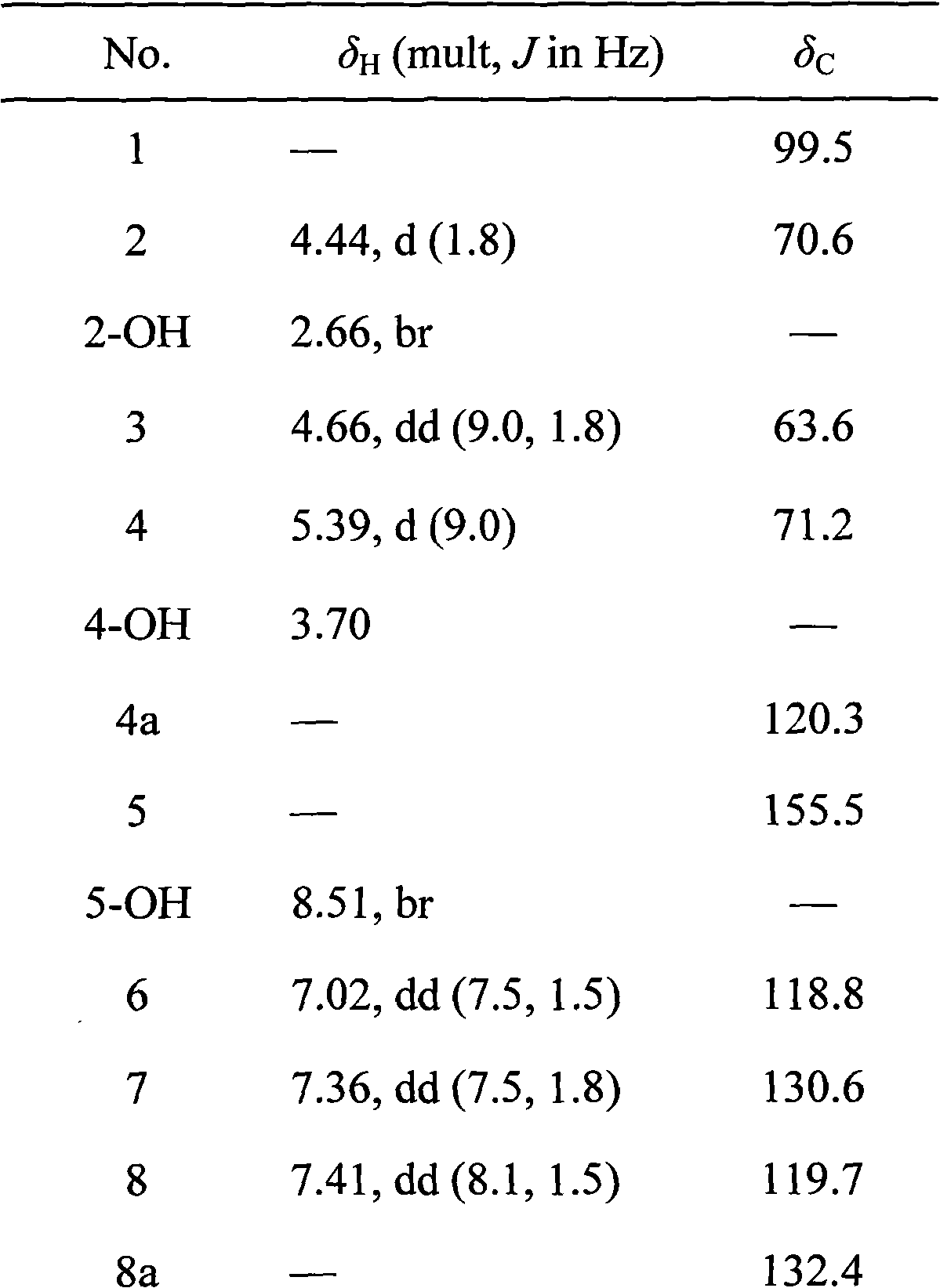
Bruguiera gymnorrhiza 5 (BG5) as well as preparation method and application thereof
A technology of palmitomycin and BG5, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, organic chemistry, etc., and can solve problems such as reports on the structure and activity of unseen compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the preparation of palmiticin BG5
[0022] 1. Extraction: Extract 4.0 kg of stems and leaves of the Chinese mangrove olive with methanol solvent, and obtain a crude extract after concentrating the obtained extract; dissolve the crude extract in water, suspend evenly, and then use ethyl acetate (3× 1.5L) and n-butanol (3×1.5L), and the resulting extract was concentrated to obtain ethyl acetate extract (85g) and n-butanol extract (25g), respectively.
[0023] 2. Separation: carry out silica gel column chromatography on ethyl acetate extract, and use petroleum ether / ethyl acetate gradient elution, wherein the volume ratio of petroleum ether / ethyl acetate in gradient elution is 100:0, 90:10, 80:20, 70:30, 60:40 and 50:50; among them, petroleum ether / diethyl ether (volume ratio 70:30) eluting part, through Sephadex LH-20 gel column chromatography, with petroleum ether / chloroform / Elution with methanol (volume ratio 2:1:1), followed by silica gel column chromat...
Embodiment 2
[0028] The test of embodiment 2 antitumor activity
[0029] 1. Experimental samples and experimental methods
[0030] Preparation of the experimental sample solution: the test sample is the compound palmiticin BG5 isolated and prepared in the above-mentioned Example 1. Accurately weigh an appropriate amount of sample, and use dimethyl sulfoxide (DMSO) to prepare a solution of the desired concentration for activity testing.
[0031] The in vitro antitumor activity of HL-60 human leukemia cells was determined by tetrazolium salt (MTT) reduction method. HL-60 human leukemia cells in the logarithmic growth phase (American Type Culture Collection, ATCC) were seeded in 96-well microculture plates at 90 μL / well (8000 cells / well), and cultured After 24 hours, 10 μL / well of the drug solution was added, and each concentration was repeated in triplicate wells, and a cell-free zero well was set up. Tumor cells were incubated at 37°C, 5% CO 2 After culturing under conditions for 72 hou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com