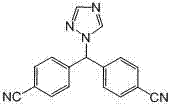
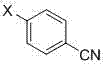
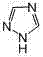Novel method for synthesizing 4,4',4''-trinitrile phenylmethane serving as letrozole-related substance
A technology for trinitrile phenylmethane and mixtures, applied in the field of letrozole-related substances 4, which can solve problems such as harsh production conditions, no compound synthesis method, and difficult separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 22.4g of potassium tert-butoxide and 0.2L of dimethylformamide into a 1L three-necked flask, cool down to -10°C, stir for 10min, add 11.7g of 4-methylbenzonitrile dropwise at this temperature and dissolve in 150ml of N,N-dimethylformamide The solution of methyl formamide, control the dropping time over 30min, dropwise, keep stirring for 30min, add dropwise the solution of 25.0g 4-fluorobenzonitrile dissolved in 150mlN,N-dimethylformamide at this temperature, dropwise , Stirring at this temperature for 4h. After the reaction is complete, add 3N hydrochloric acid to extract it, adjust the pH to neutral, stir for 30 minutes, concentrate under reduced pressure until no liquid is evaporated to obtain a reddish-brown oil, add 500ml of water and 500ml of ethyl acetate to the concentrate, stir for 10min, and divide liquid, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain a reddis...
Embodiment 2
[0040] Add 22.4g of potassium tert-butoxide and 0.2L of N,N-dimethylformamide into a 1L three-necked flask, cool down to -10°C, stir for 10min, add 11.7g of 4-methylbenzonitrile dropwise at this temperature and dissolve in 150ml of N,N -The solution of dimethylformamide, control the dropping time over 30min, after dropping, keep stirring for 30min, then add dropwise the solution of 25.0g of 4-fluorobenzonitrile dissolved in 150ml of N,N-dimethylformamide at this temperature , after dropping, the temperature was naturally raised and stirred overnight. After the reaction is complete, add 3N hydrochloric acid to extract it, adjust the pH to neutral, stir for 30 minutes, concentrate under reduced pressure until no liquid is evaporated to obtain a reddish-brown oil, add 500ml of water and 500ml of ethyl acetate to the concentrate, stir for 10min, and divide liquid, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under ...
Embodiment 3
[0042] Add 33.6g of potassium tert-butoxide and 0.2L of N,N-dimethylformamide into a 1L three-necked flask, cool down to -10°C, stir for 10min, and add dropwise a solution of 11.7g of 4-methylbenzonitrile dissolved in 150ml of DMF at this temperature , control the dropping time over 30min, after dropping, keep stirring for 30min, add dropwise 25.0g of 4-fluorobenzonitrile dissolved in 150ml of N,N-dimethylformamide solution at this temperature, after dropping, naturally heat up and stir overnight . After the reaction is complete, add 3N hydrochloric acid to extract it, adjust the pH to neutral, stir for 30 minutes, concentrate under reduced pressure until no liquid is evaporated to obtain a reddish-brown oil, add 500ml of water and 500ml of ethyl acetate to the concentrate, stir for 10min, and divide liquid, and the organic phase was dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain a reddish-brown oily liqui...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com