Process for preparing high purity letrozole
A technology for letrozole and a compound is applied in the field of preparation of high-purity letrozole, can solve the problems of no effective refining intermediate, complicated and tedious operation, and high production cost, and achieves the operation of avoiding column chromatography and multiple recrystallizations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 high-purity letrozole
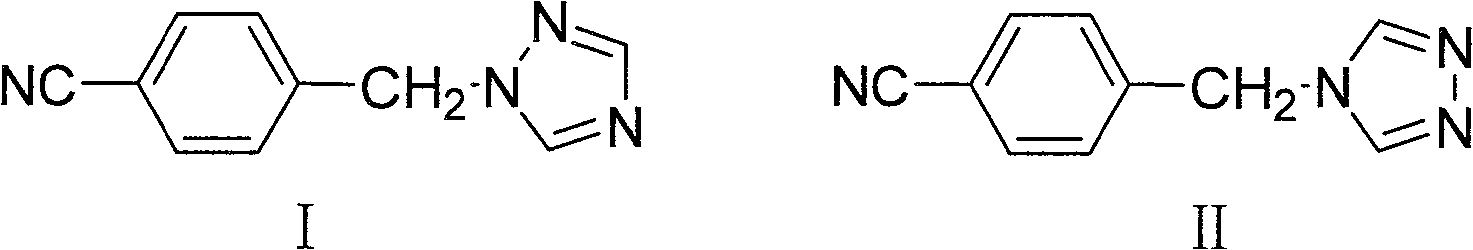
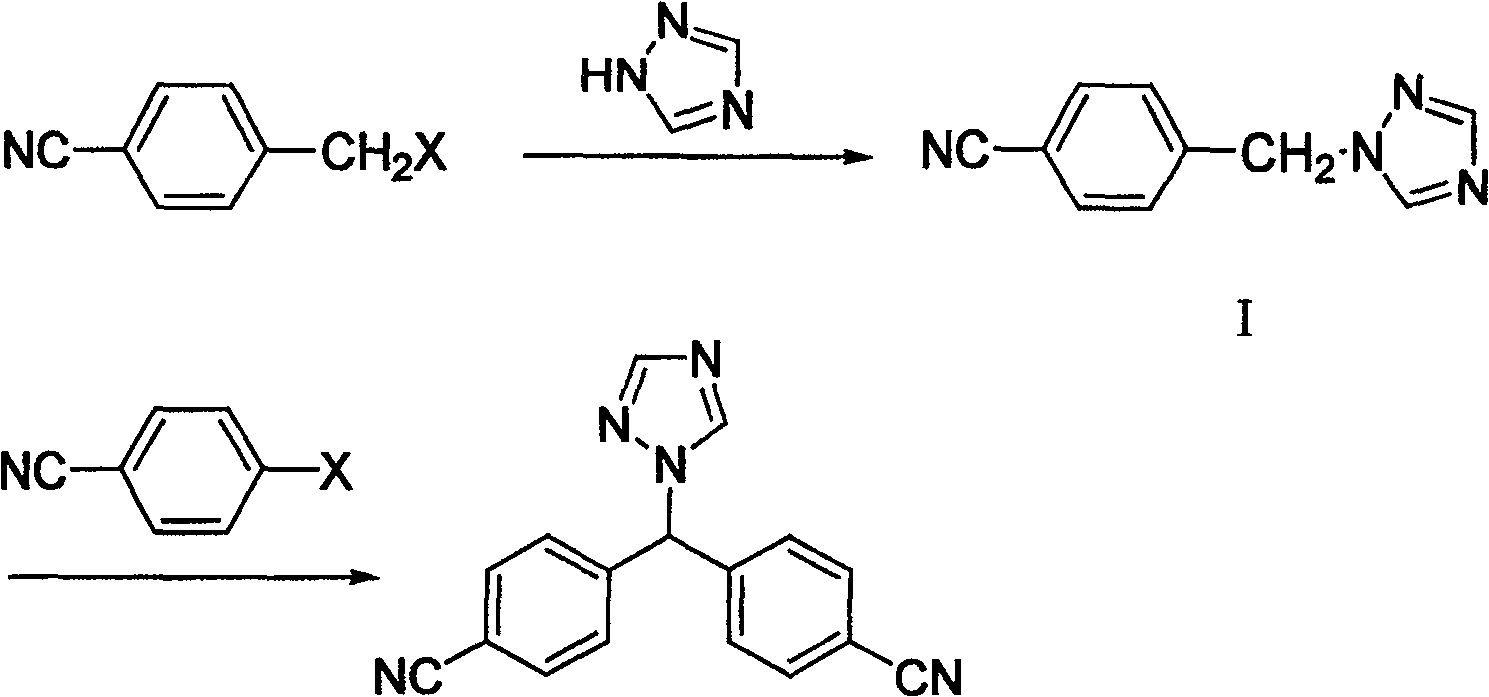
[0023] Add 1 liter of chloroform into a 2-liter reaction flask, add 60 grams of p-cyanobenzyl bromide and 90 grams of 1,2,4-triazole under stirring, and heat to reflux for 20 hours. Cool, wash with 5% sodium bicarbonate solution, and dry the organic phase. After filtration, the filtrate was evaporated to dryness under reduced pressure to obtain about 82 grams of a mixture of the intermediate compound of formula I and its isomer compound of formula II.
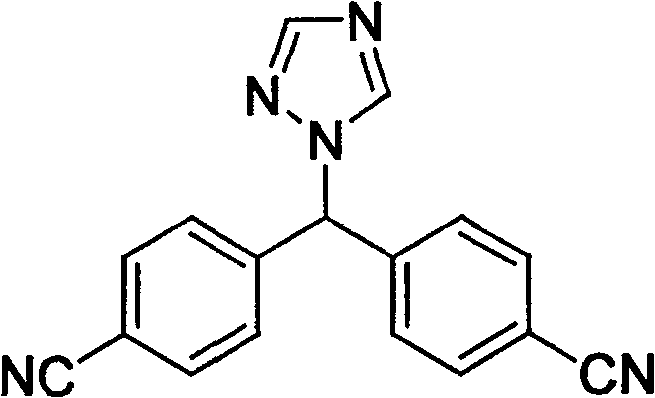
[0024] 82 g of the mixture obtained above (the ratio of the compound of formula I to the compound of formula II is about 2:1) was dissolved in 500 ml of ethanol, and about 30 ml of concentrated hydrochloric acid was slowly added dropwise with stirring, and the precipitate was removed by filtration. Continue to add concentrated hydrochloric acid to the filtrate until no more precipitates are formed. Filtration yielded 74 g of the hydrochloride salt of the compound ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com