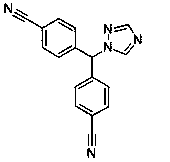
Letrozole dispersing tablet dosage form
A technology of letrozole and dispersible tablets, applied in the field of medicine, can solve the problems of large gastrointestinal irritation, low bioavailability, inappropriateness and the like, and achieves the effects of low production and packaging cost, simple production process and convenient taking.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 (prescription quantity is 1000 tablets)
[0018] Prescription A:
[0019] Letrozole 2.5g
[0020] Microcrystalline cellulose (disintegrant) 122.5g
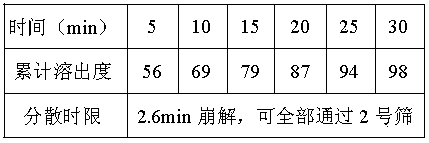
[0021] Preparation process: The main drug is passed through a 100-mesh sieve, and the disintegrant is passed through a 80-mesh sieve. Weigh the prescribed amount of the disintegrant and mix evenly. , granulated, dried and pressed into tablets. Dispersible tablet dispersion time limit is 2.6min.
Embodiment 2
[0022] Embodiment 2 (prescription quantity is 1000 tablets)
[0023] Prescription B:
[0024] Letrozole 2.5g
[0025] Lactose (filler) 127g
[0026] Sodium carboxymethyl starch (disintegrant) 6g
[0027] 4%PVP K3Q Absolute ethanol solution (adhesive) 50g
[0028] Magnesium stearate (lubricant) 1g
[0029] Preparation process: the main drug is passed through a 100-mesh sieve, the filler and disintegrant are passed through a 80-mesh sieve, and the prescribed amount of filler and disintegrant is weighed and mixed evenly, and then the prescribed amount of the main drug is mixed with it by an equal amount of incremental method , add an appropriate amount of binder to granulate, add the prescribed amount of lubricant after drying, mix well, and press into tablets. Dispersible tablet dispersion time limit is 2.1min.
Embodiment 3
[0030] Embodiment 3 (prescription quantity is 1000 tablets)
[0031] Prescription C:
[0032] Letrozole 2.5g
[0033] Microcrystalline cellulose (filler) 200g
[0034] Cross-linked polyvinylpyrrolidone (disintegrant) 12g
[0035] 4%PVP K3Q Absolute ethanol solution (adhesive) 45g
[0036] Magnesium stearate (lubricant) 2g
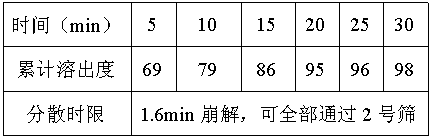
[0037] The preparation process is the same as that of prescription B. The disintegration time limit of dispersible tablet is 1.6min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com