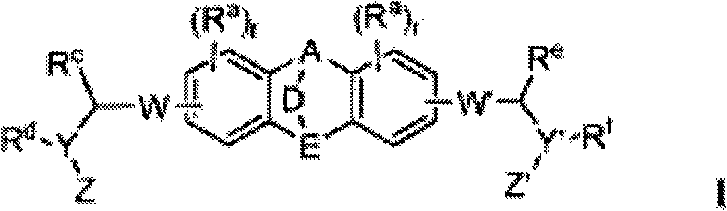
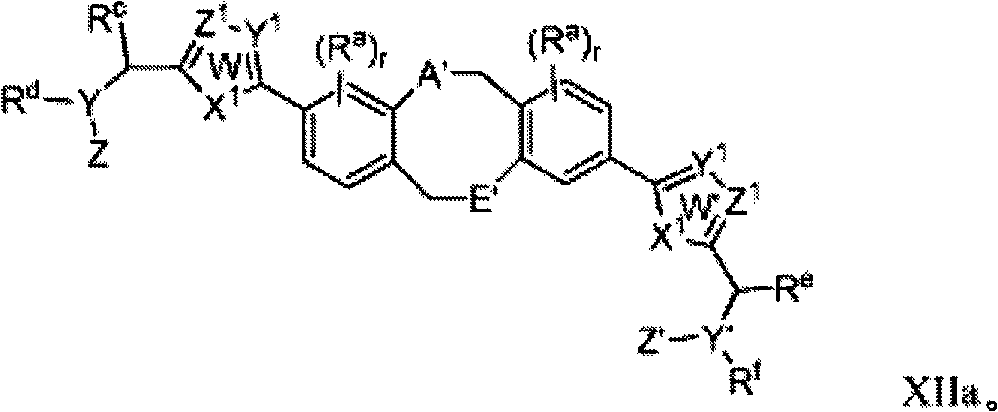
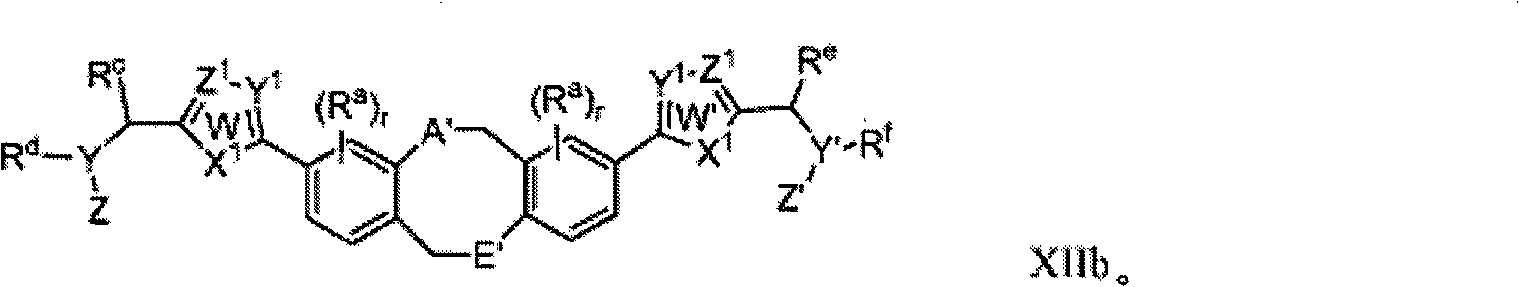
Fused ring inhibitors of hepatitis c
一种杂环、环烷基的技术,应用在非结构性5A蛋白功能的化合物领域,能够解决副作用、致衰弱、治疗作出反应等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11-5
[0903] Example 1.1-5, (2S,2′S)-1,1′-((2S,2′S)-2,2′-(5,5′-(dibenzo[b,e][1 , 4] Dioxin-2,7-diyl)bis(1H-imidazole-5,2-diyl))bis(pyrrolidine-2,1-diyl))bis(3-methyl Of dimethyl-1-oxobutane-2,1-diyl) dicarbamate
[0904] Step 1. At -78 ℃, dibenzo-p-dioxin (1-1) (5.0g, 27.14mmol) and chloroacetyl chloride (4.5mL, 57mmol) in dichloromethane (50mL) The solution was added to a stirred suspension of aluminum chloride (14.5 g, 108.6 mmol) in dichloromethane (300 mL) over 20 minutes, and the reaction mixture was stirred at -78°C for 15 minutes and allowed to warm up over 30 minutes To room temperature. The reaction mixture was then heated at 50°C for 3 hours and stirring was continued overnight at room temperature. The reaction was cooled to 0°C and carefully quenched with ice-cold water (250 mL). The volatiles were removed in vacuo, the formed precipitate was collected by vacuum filtration, washed with diethyl ether, and dried under vacuum at 50°C to obtain the crude product 1-2 (8.85g,...
Embodiment 2
[0909] Preparation of Example 2.1-4B
[0910]
[0911] Follow the general procedure A described above for the synthesis of 1-4, and replace N-Boc-L-proline with N-Boc-L-pipecolic acid in step a to obtain the compound in a yield of 60% 1-4B (0.82g). LC-MS(ESI): m / z 681[M-H] - .
Embodiment 3
[0912] Preparation of Example 3.1-5B
[0913]
[0914] Following general procedure B, and replacing 1-4A with compound 1-4B, compound 1-5B is obtained. LC-MS(ESI): m / z 797[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com