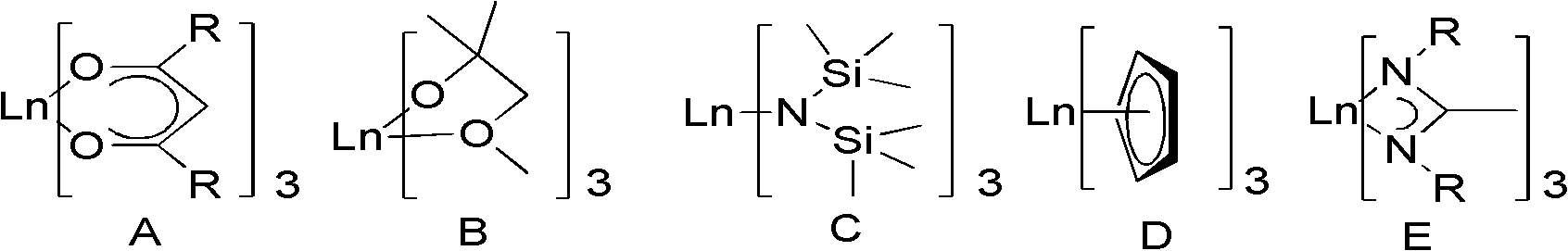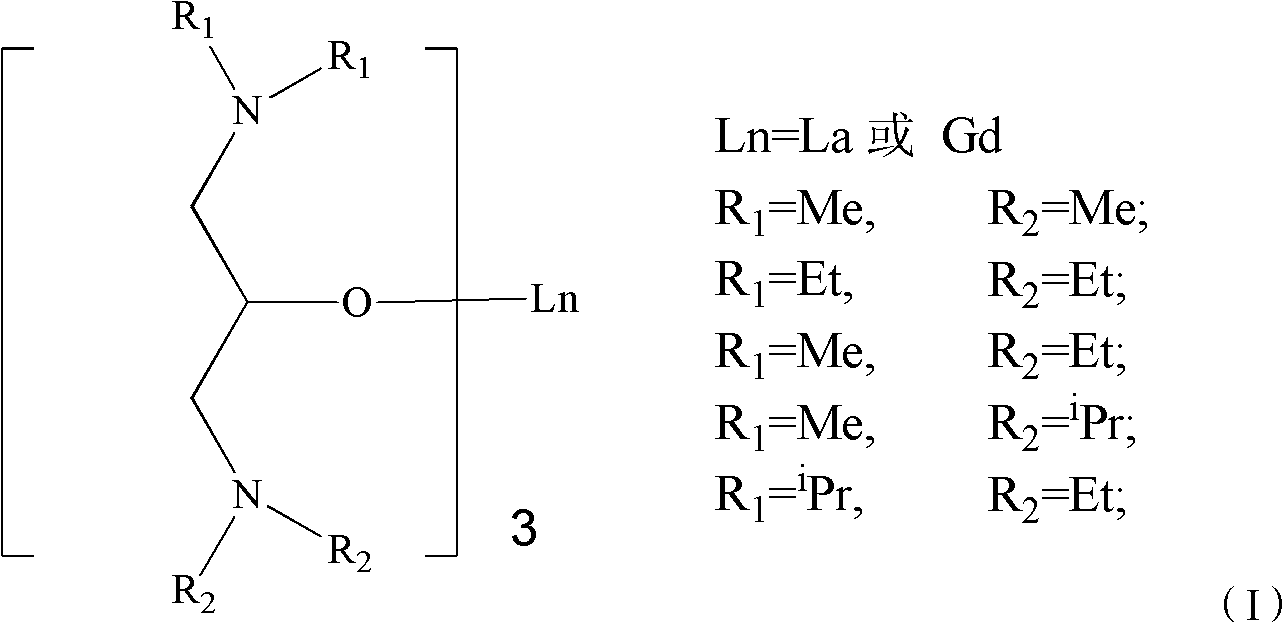Nitrogen-containing functional group substituted alkoxy rare-earth metal lanthanum and gadolinium complex and synthetic method and application thereof
A nitrogen-containing functional group and alkoxy rare earth technology, applied in the application field of ALD precursors in the preparation of high-K materials, to achieve the effects of good flatness, easy preparation, and reasonable price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
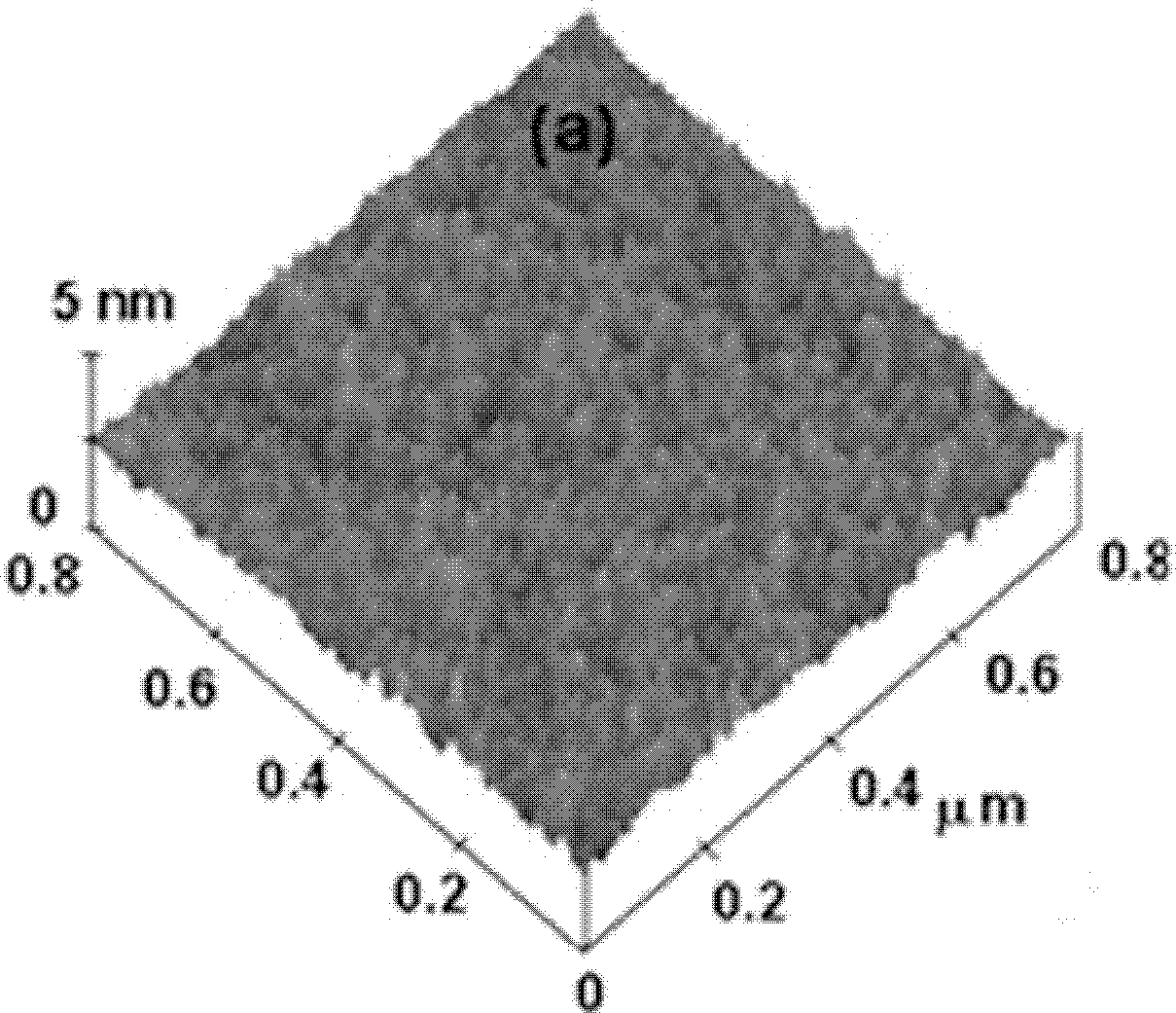
Image
Examples
Embodiment 1
[0029] Example 1 La{OCH[CH 2 N(CH 3 ) 2 ] 2} 3 Synthesis of complexes.
[0030] After removing water and oxygen from the reaction bottle, the inert gas N 2 protection, 1.2080g La[N(SiMe 3 ) 2 ] 3 0.8546 g of 1,3-bis(dimethylamine) isopropanol solution dissolved in THF was slowly added dropwise to the THF solution, returned to 30° C. and stirred for 12 hours, and the solution was clear and transparent. The solvent was drained, extracted with toluene to obtain a filtrate, and drained to obtain a white powder. Yield: 90% (1.006 g, 1.75 mmol).Anal.Calc.for C 33 h 75 GdN 6 o 3 : C, 52.07; H, 9.93; N, 11.04; Found: C, 52.10; H, 9.90, N, 11.03. 1 H NMR (500MHz, C 6 D. 6 ): δ 4.12 (m, 1H, OCH), 2.796 (s, 12H, CH 2 / N(CH2 CH 3 ) 2 ), 2.456 (m, 4H, NCH 2 ), 1.134(t, 12H, CH 3 / N(CH 2 CH 3 ) 2 ). 13 C NMR (300MHz, C 6 D. 6 ): δ 71.60 (OCH), 68.57 (NCH 2 ), 47.14 (N (CH 3 ) 2 ).
Embodiment 2
[0031] Example 2 Gd{OCH[CH 2 N(CH 3 ) 2 ] 2} 3 Synthesis of complexes
[0032] After removing water and oxygen from the reaction bottle, the inert gas N 2 protection, 0.9231g Gd[N(SiMe 3 ) 2 ] 3 0.6185 1,3-bis(dimethylamine) isopropanol solution dissolved in THF was slowly added dropwise into the THF solution, returned to 25° C. and stirred for 16 h, the solution was clear and transparent. The solvent was drained, extracted with toluene to obtain a filtrate, and drained to obtain a white powder. Yield: 92% (0.7879g, 1.33 mmol).Anal.Calc.for C 18 h 42 LaN 3 o 3 : C, 42.54; H, 8.67; N, 14.17; Found: C, 42.50; H, 8.69; N14.16.
Embodiment 3
[0033] Example 3 La{OCH[CH 2 N(CH 2 CH 3 ) 2 ] 2} 3 Synthesis of complexes
[0034] After removing water and oxygen from the reaction bottle, the inert gas N 2 protection, 1.583g La[N(SiMe 3 ) 2 ] 3 A solution of 1.549 g of 1,3-bis(diethylamine)isopropanol dissolved in toluene was slowly added dropwise into the toluene solution, and the temperature was returned to 25° C. and stirred for 12 hours, and the solution was clear and transparent. The solvent was removed, and the filtrate was obtained by extraction with n-hexane, which was dried to obtain a colorless oily liquid. Yield: 90% (1.748g, 2.296 mmol).Anal.Calc.for C 33 h 75 LaN 6 o 3 : La, 26.23; C, 47.63; H, 9.14, Found: La, 26.20; C, 47.65; H, 9.15. 1 H NMR (300MHz, C 6 D. 6 ): δ 3.69 (m, 1H, CHO), 2.37 (m, 4H, CH 2 / N(CH 2 CH 3 ) 2 ), 2.04 (d, J=5.8 Hz, 2H, CH 2 N), 1.14(d, J=5.7 Hz, 3H, CH 3 / CH 3 CH), 1.02(t, J=7.0 Hz, 6H, CH 3 / N(CH 2 CH 3 ) 2 ). 13 C NMR (300MHz, C 6 D. 6 ): δ 72.32 (CHO...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com