Kanamycin antisense nucleic acid for the treatment of cancer
A technology of kanamycin and nucleic acid, applied in the direction of antibody medical ingredients, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problems of maintenance treatment compliance recurrence, lack of patients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1D
[0127] Example 1 DNA construct
[0128] The 5 DNA constructs used:
[0129] -pCDNA 3 The PML-RARαFrC construct (also known as VVACS01 ) is described in Example 1b on page 18, line 12 to page 19, line 2 of WO 03 / 090778.
[0130] -pCDNA 3 The PML-RARαASFrC construct (also known as VVACS04) is described in Example 1b on page 19, lines 4-15 of WO 03 / 090778.
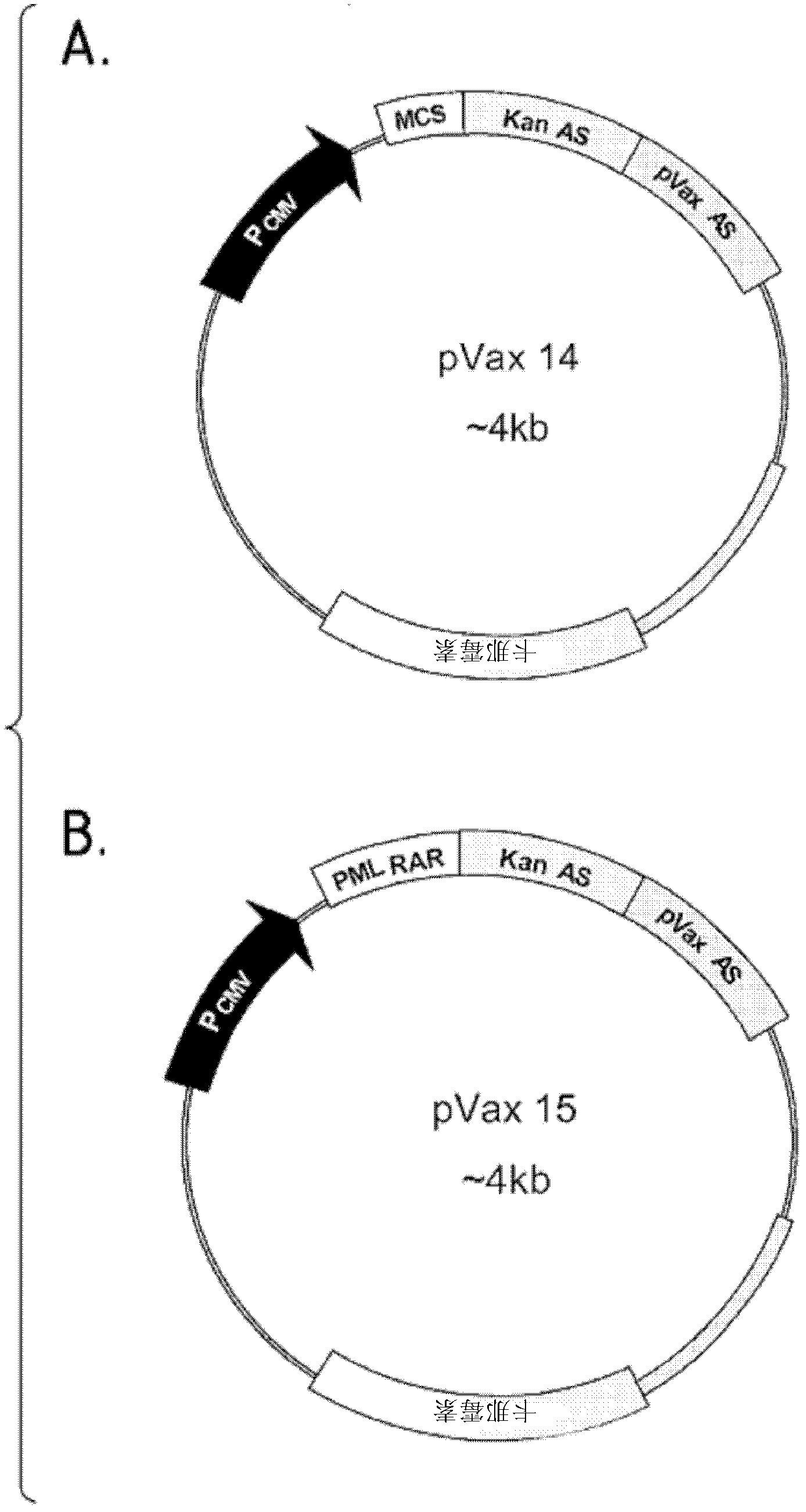
[0131] - The pVaxKanAS construct (also known as pVax14 or VVACS02) obtained by cloning a nucleic acid consisting of the sequence shown in SEQ ID NO: 1 into the pVax1 vector.
[0132] - pVaxPML-RARα KanAS construct (also known as pVax15 or VVACS03), obtained by cloning the coding sequence of the PML-RARα tumor antigen into pVax14.
[0133] - pVaxPML-RARαFrC construct obtained by cloning PML-RARαFrC into pVax1 vector.
[0134] pVax14 (pVaxKanAS or VVACS02) was constructed by PCR amplification of pVax1 by using the following primer pairs: the primer pair shown in SEQ ID NOs: 10 and 11 and the primer pair shown in SEQ ID NOs...
Embodiment 2
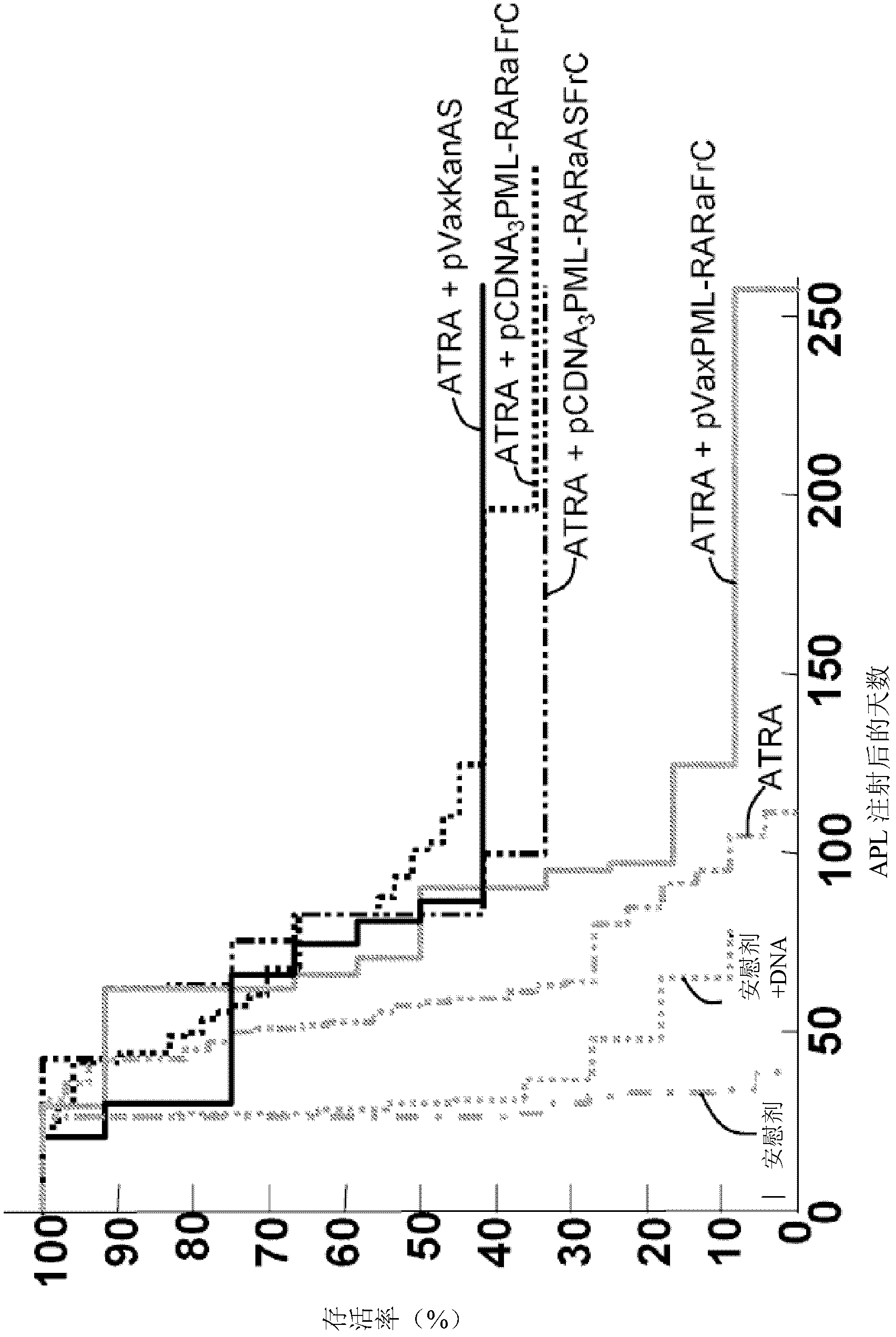
[0137] Example 2 KanAS prolongs the lifespan of APL model mice
[0138] The effect of different DNA constructs on APL model mice was studied using the procedure disclosed by Padua et al. (2003, Nature Medicine 9: 1413-1417). Simply put, 10 4 APL cells were injected into FVB / N mice. An ATRA pellet capable of releasing 5 mg ATRA per day for 21 days was then introduced into the back of the mouse's neck via a trochar. DNA (2 x 50 micrograms of plasmid) was injected one day after the introduction of the ATRA pellet, for a total of three injections every 20 days. Mice were injected with the following DNA constructs:
[0139] - pVaxKanAS construct, also known as pVax14;
[0140] -pCDNA 3 PML-RARαFrC construct (VVACS01);
[0141] - pVaxPML-RARαFrC construct; and
[0142] -pCDNA 3 PML-RARαASFrC construct (VVACS04).
[0143] Pellets purchased from the ATRA pellet manufacturer (Innovative Research, Sarasota, USA) were used as placebo.
[0144] Mice were followed for 2 years and...
Embodiment 3
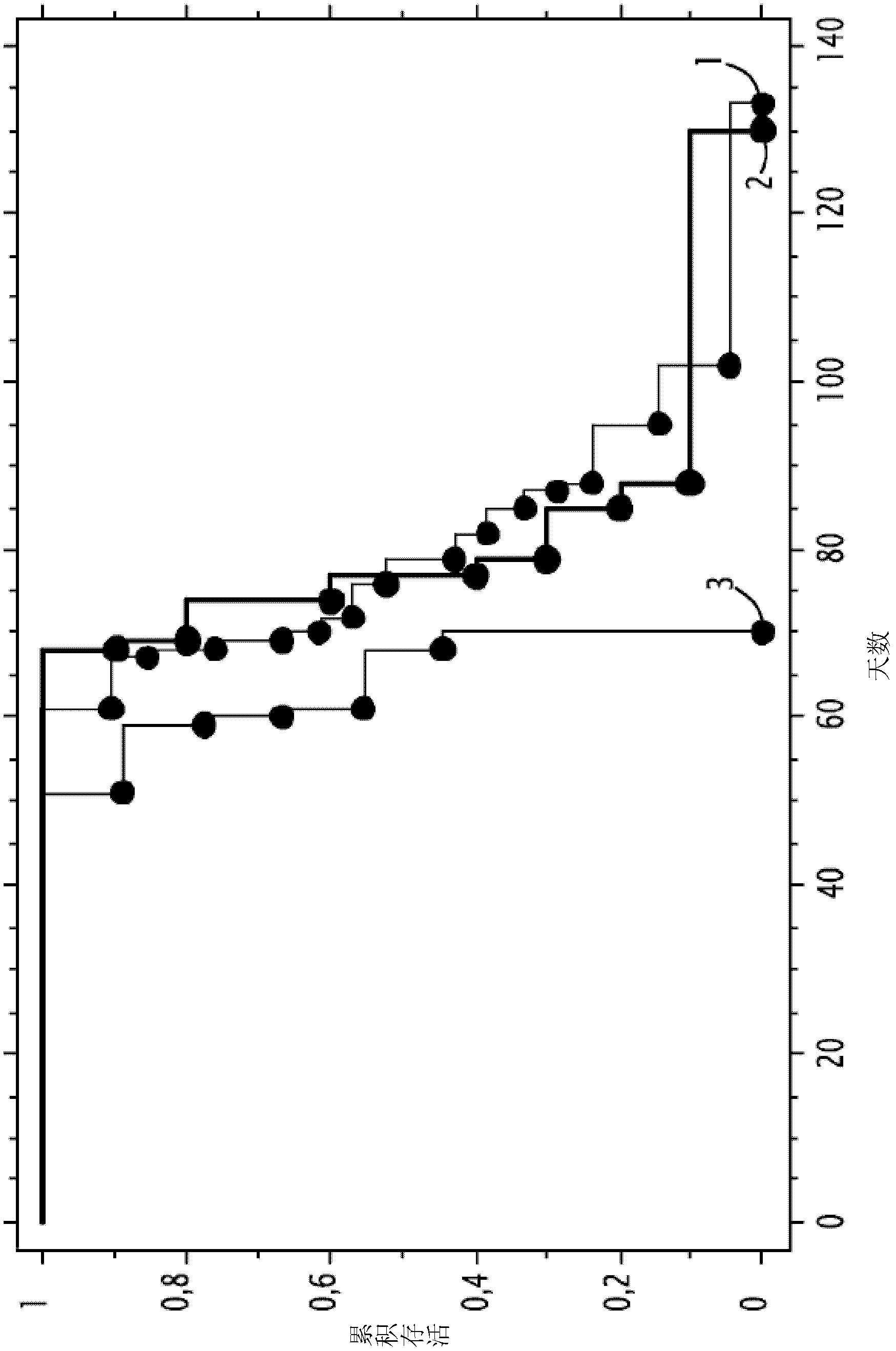
[0155] Example 3 KanAS and PMLRARαASFrC prolong the lifespan of MDS model mice
[0156] The MDS mouse model described by Omidvar et al. (2007, Cancer Res. 67: 11657-67) was used to study the role of KanAS in MDS. The mouse model is based on NRAS mutant mice with BCL-2, which are followed until their platelet counts drop, which is indicative of thrombocytopenia and imminent death.
[0157] The inoculation steps are as follows: on the day after the diagnosis (day 0), the corresponding peripheral blood platelet count drops to the normal value (5 / μl) or less, 2×50 μg of DNA was administered. On days 20 and 40, 2 x 50 micrograms of DNA were injected again.
[0158] The first experiment was performed by treating 6 mice with pVaxKanAS (VVACS02). Such as Figure 4 As shown, KanAS alone prolonged the lifespan of mice compared to the no treatment group (p=0.003).
[0159] Treatment of 10 mice with pVaxKanAS and pCDNA 3 The second experiment was performed by treating 16 mice with P...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com