Novel lactim compound possessing cardiotonic action, its preparation method and its purpose
A technology of lactim and compounds, which is applied in the field of lactim compounds, can solve the problems such as status reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1, the preparation method of matsutake ethyl acetate extract
[0025] 2 kg of dry matsutake (Tricholoma Matsutake Sing) was soaked in water or 0-70% (ethanol-water, v / v) ethanol at room temperature for 5-8 weeks, and the obtained ethanol extract was dispersed in water and extracted with ethyl acetate. The ethyl acetate extract was recovered under reduced pressure to obtain 8 g of the ethyl acetate layer sample.
Embodiment 2
[0026]Example 2. Separation and purification of lactam monomer compounds in matsutake ethyl acetate extract Instrument materials: high performance liquid chromatography: HPLC detector-Shimadzu RID-6A refractive index; chromatographic column: 5C18-MS-II, 4.6× 250mm. Silica gel for column chromatography (200-300 mesh), silica gel for thin layer chromatography GF 254 (10-40 μm) (manufactured by Qingdao Ocean Chemical Co., Ltd.), ODS for column chromatography: DM1020T (100-200 mesh).
[0027] Separation and purification process: the ethyl acetate layer sample (prepared in Example 1) was subjected to silica gel column chromatography, and gradient elution was performed with cyclohexane-ethyl acetate and chloroform-methanol respectively to obtain 8 fractions (Fr.1-8 ), wherein Fr.8 was eluted by ODS column chromatography with methanol-water (10%~80%, v / v) as the eluent for gradient elution, wherein Fr.8-2 was separated by HPLC preparative chromatography, and methanol-water Water wa...
Embodiment 3
[0028] Embodiment 3, structure analysis of formula I compound
[0029] Instruments and equipment: polarimeter: Perkin-EImer 241 polarimeter; mass spectrometer: JEOL GCmate, Bruker APEX micrOTOF-Q; nuclear magnetic resonance: JNM-LA500 (500MHz).
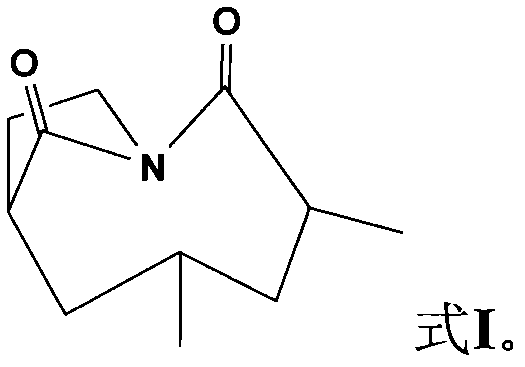
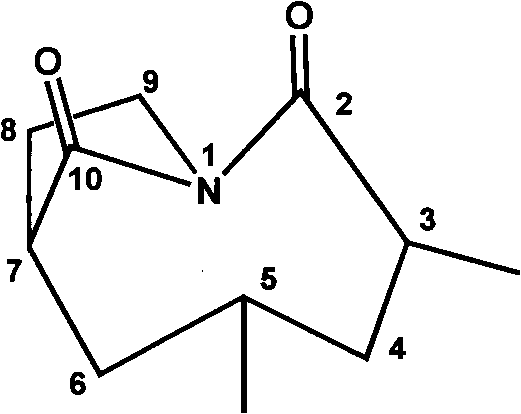
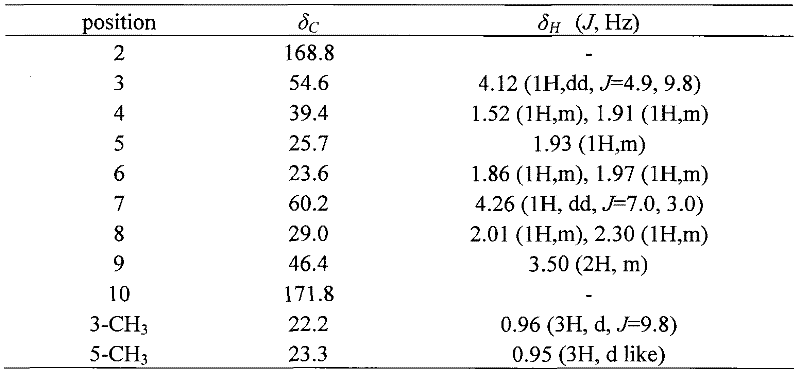
[0030] Compound I yellow oil, (c 0.72, CHCl 2 ), HR-EI-MS: 195.1261[M]+, the molecular formula is C 11 h 17 NO 2 . According to its physical and chemical properties and spectroscopic data, this compound was identified as 3,5 dimethyl-1-nitrobicyclo[5,2,1]decane-2,10-dione (3,5-dimethyl-1- azabicyclo[5,2,1]decane-2,10-diketone), a new compound that has not been reported in the literature. Its NMR (500MHz, CD 3 OD) data are shown in Table 1.
[0031]
[0032] Formula I compound structural formula
[0033] Table 1, formula I compound 1 H. 13 C NMR data attribution
[0034]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com