Preparation method of FeOCl
A ferric oxychloride, fully grinding technology, used in chemical instruments and methods, iron compounds, inorganic chemistry, etc., can solve problems such as nitrogen waste, and achieve the effect of pure phase and good crystal shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] a. Take a certain amount of FeCl 3 ·6H 2 O, put into an agate mortar and grind thoroughly, transfer to a crucible.
[0011] b. Put the above substances in an oven, hydrolyze at 220°C for 4 hours, and cool down to room temperature naturally.
[0012] c. Then take out the crucible, put the sample in a mortar and grind it thoroughly, rinse it with a large amount of acetone to remove the unreacted FeCl 3 ·6H 2 0, then put it into a vacuum drying oven for vacuum drying at 80°C.
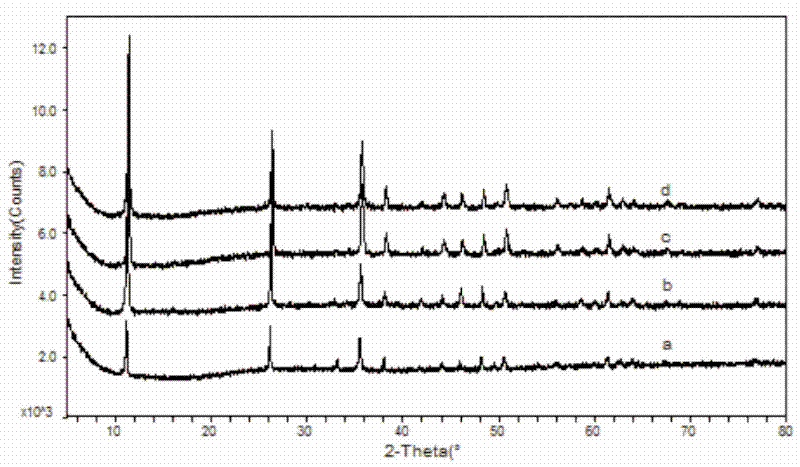
[0013] The XRD figure of the ferric oxychloride that the present embodiment obtains is figure 1 In a, it can be seen from the figure that the obtained sample has a pure phase and a good crystal form.
Embodiment 2
[0015] a. Take a certain amount of FeCl 3 ·6H 2 O, put into an agate mortar and grind thoroughly, transfer to a crucible.
[0016] b. Put the above substances in an oven, hydrolyze at 230°C for 2.5 hours, and cool down to room temperature naturally.
[0017] c. Then take out the crucible, put the sample in a mortar and grind it thoroughly, rinse it with a large amount of acetone to remove the unreacted FeCl 3 ·6H 2 0, then put it into a vacuum drying oven for vacuum drying at 80°C.
[0018] The XRD figure of the ferric oxychloride that the present embodiment obtains is figure 1 In b, it can be seen from the figure that the obtained sample has a pure phase and a good crystal form.
Embodiment 3
[0020] a. Take a certain amount of FeCl 3 ·6H 2 O, put into an agate mortar and grind thoroughly, transfer to a crucible.
[0021] b. Put the above substances in an oven, hydrolyze at 240°C for 90 minutes, and cool down to room temperature naturally.
[0022] c. Then take out the crucible, put the sample in a mortar and grind it thoroughly, rinse it with a large amount of acetone to remove the unreacted FeCl 3 ·6H 2 0, then put it into a vacuum drying oven for vacuum drying at 80°C.
[0023] The XRD figure of the ferric oxychloride that the present embodiment obtains is figure 1 In c, it can be seen from the figure that the obtained sample has a pure phase and a good crystal form.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com