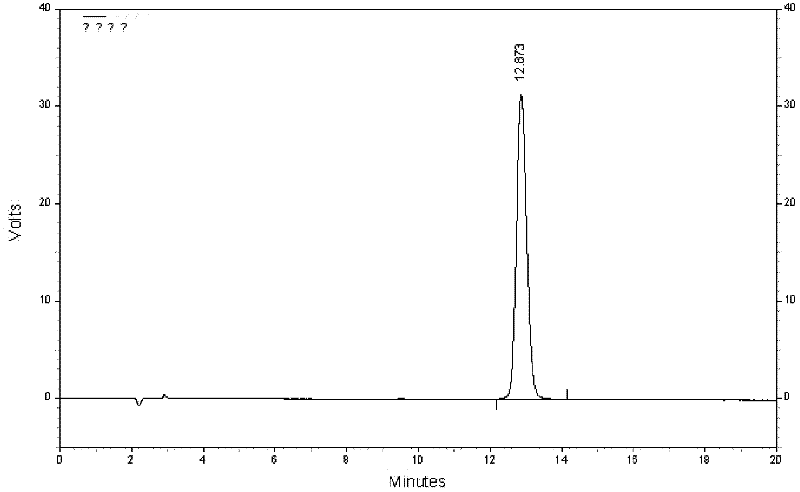
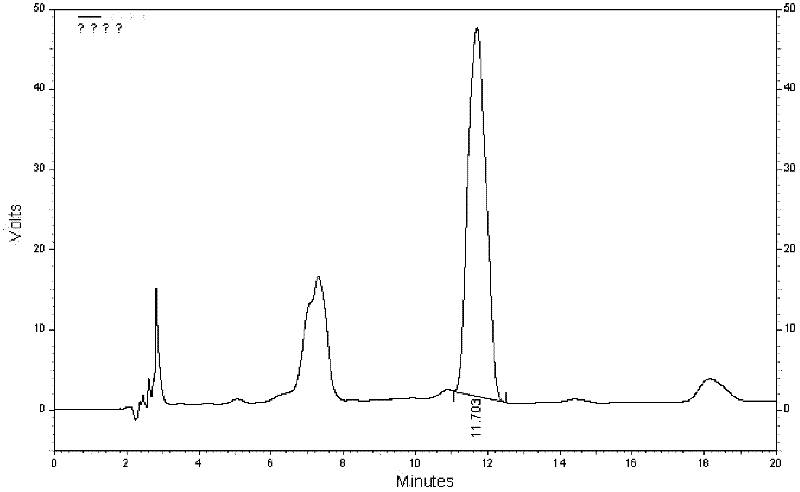
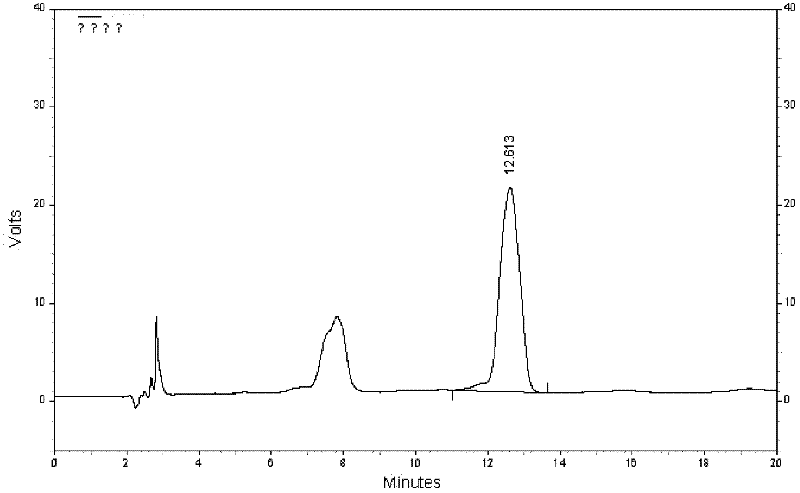
Quality control method for eight-flavor agilawood preparations
A kind of eight-flavor agarwood and agarwood technology, which is applied in the field of medicine, can solve the problems of poor patient taking compliance, low number of medicinal materials for thin-layer identification, production technology, and quality standards to be improved, etc., and achieves the effect of improving sensitivity and product quality and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Take 100g of agarwood, 100g of nutmeg, 100g of jujube, 100g of myrobalan, 50g of frankincense, 175g of woody wood, 75g of kapok, and 50g of travertine; The mass percentage is 5%; the remaining medicinal materials, jujube, chebula, kapok, and travertine are dried under reduced pressure at 70°C and the moisture is controlled to be 6% in mass percentage; Mesh sieve, and then mix evenly, water pills, dry, that is.
Embodiment 2
[0223] The preparation of embodiment 2. Bawei agarwood capsules
[0224] Get 100g of agarwood, 100g of nutmeg, 100g of jujube, 100g of myrobalan, 50g of frankincense, 175g of woody wood, 75g of kapok, and 50g of travertine; according to the conventional process, add conventional auxiliary materials to prepare capsules.
Embodiment 3
[0225] The preparation of embodiment 3. Bawei agarwood granules
[0226] Get 100g of agarwood, 100g of nutmeg, 100g of jujube, 100g of myrobalan, 50g of frankincense, 175g of woody wood, 75g of kapok, and 50g of travertine; according to the conventional process, add conventional auxiliary materials to prepare granules.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com