Low-osmotic-pressure triiodo-benzene compound contrast agent
A kind of compound and contrast agent technology, applied in the field of non-ionic triiodobenzene compound contrast agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
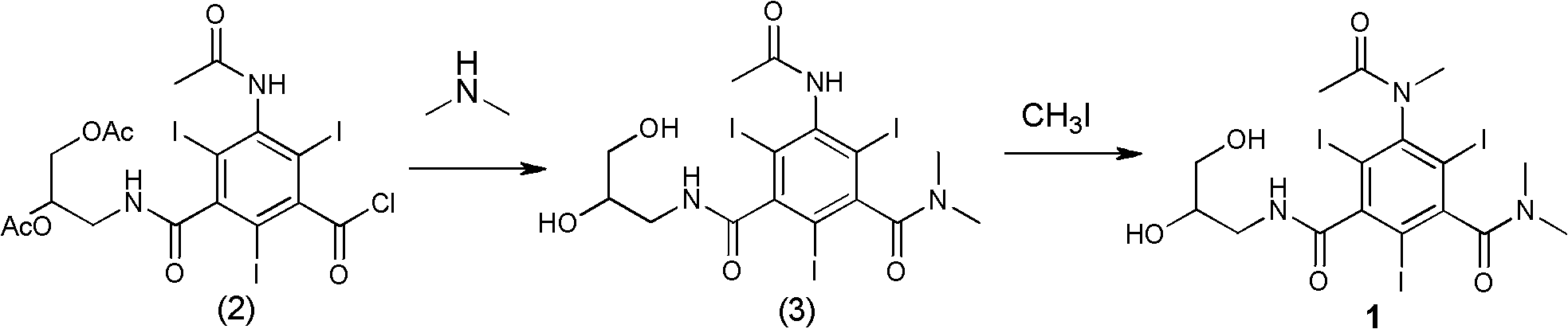
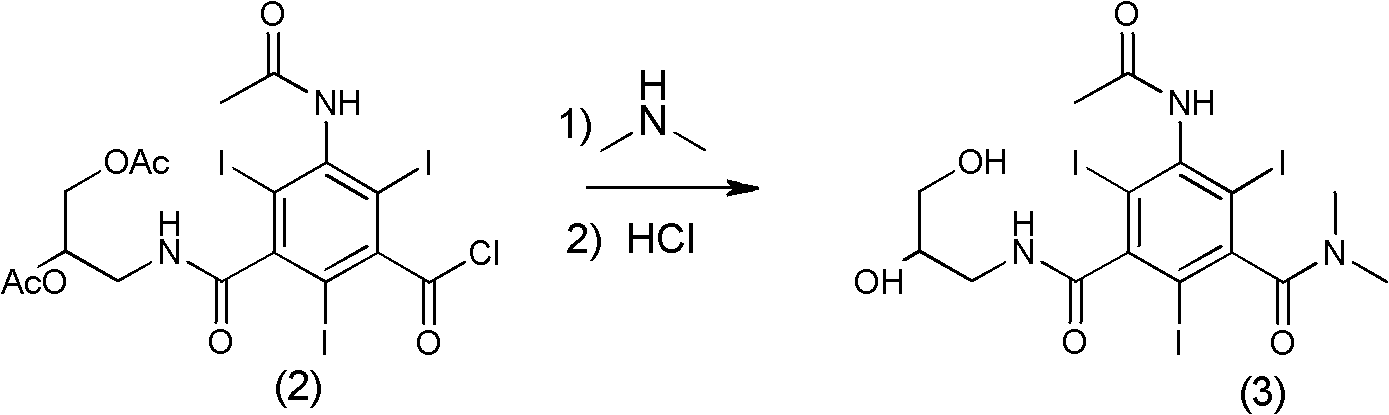
[0031] Example 1 Formula (3) compound N, N-dimethyl-N'-(2,3-dihydroxy n-propyl)-5-acetamido-2,4,6-triiodo-1,3- Synthesis of Isophthalamide
[0032]
[0033]Formula (2) compound 3-acetamido-5-(2,3-diacetoxy n-propylcarbamoyl)-2,4,6-triiodobenzoyl chloride (70g, 0.09mol), tetrahydrofuran ( 210ml) into a 1L three-necked bottle. After cooling to 0°C in an ice-water bath, an aqueous solution of dimethylamine (86.2 g, 0.63 mol, 33%) was added dropwise. After dropping (1 h), the ice-water bath was removed, and the reaction was allowed to rise to room temperature naturally, and the reaction was stirred for 48 h (the completion of the reaction was tracked by HPLC). Adjust the pH to 7 with concentrated hydrochloric acid, and concentrate under reduced pressure to obtain a yellow viscous substance. The viscous obtained was separated and purified through LX18 macroporous adsorption resin, the main component eluate was collected, treated with activated carbon, and filtered. The filtr...
Embodiment 2
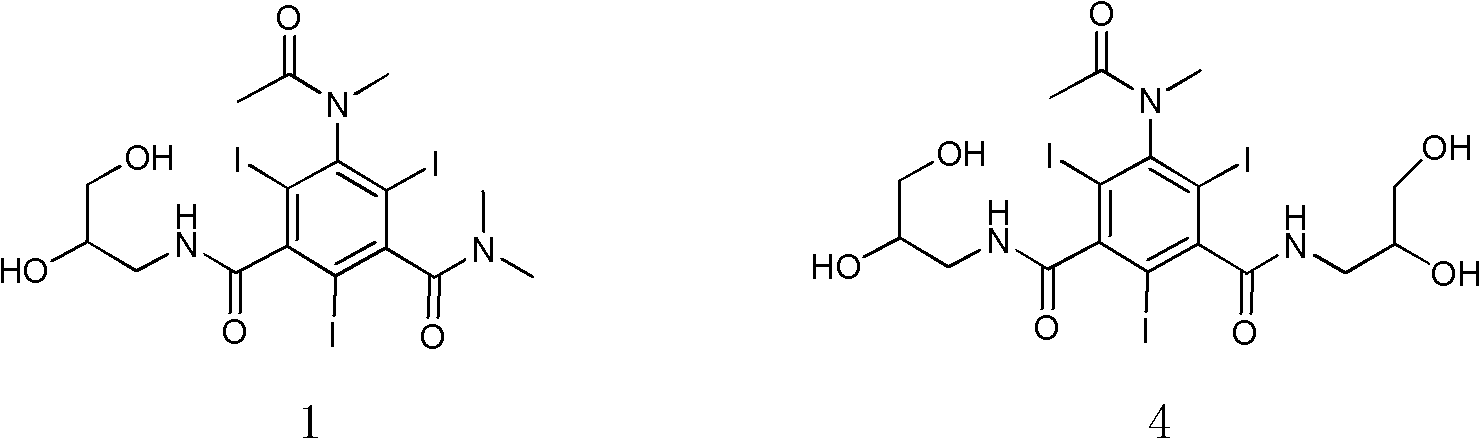
[0034] Example 2 Compound 1N, N-dimethyl-N'-(2,3-dihydroxy-n-propyl)-5-(N-methylacetamido)-2,4,6-triiodo-1, Synthesis of 3-isophthalamide
[0035]
[0036] Formula (3) compound N,N-dimethyl-N'-(2,3-dihydroxy n-propyl)-5-acetamido-2,4,6-triiodo-1,3-m-benzene Diformamide (42.3g, 0.06mol), sodium hydroxide (3.1g, 0.08mol), water (40ml), ethylene glycol monomethyl ether (160ml) were added to a 0.5L three-necked bottle, and stirred thoroughly for 30min (dissolving clear). To the reaction mixture was slowly added iodomethane (10.9 g, 0.08 mol) dropwise at room temperature. After dripping (1h). The reaction was stirred at room temperature 25°C for 21h. At room temperature, iodomethane (11 g, 0.08 mol) was continuously added dropwise to the reaction mixture slowly, and the drop was completed (1 h). Sodium hydroxide (3.1 g, 0.08 mol) was added, and the reaction was continued at room temperature (HPLC detection end point), and the reaction was completed (8h). Adjust the pH of t...
Embodiment 3
[0038] Example 3 Injection 1
[0039] Prescription 1 (300mgI / ml)
[0040]
[0041] Prescription 2 (350mgI / ml)
[0042]
[0043] Preparation:
[0044] Take the prescribed amount of compound 1, 10.0 mg of disodium calcium salt of ethylenediamine tetracarboxylic acid, and 120.0 mg of trishydroxymethylaminomethane in a 100ml volumetric flask, add water for injection to dissolve, adjust the pH to neutral, dilute to 100ml, and use a microporous Filter through a filter membrane, seal in a 10ml bottle, sterilize at 120°C for 20 minutes, take it out and cool it down to room temperature to obtain injections with different iodine contents.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com