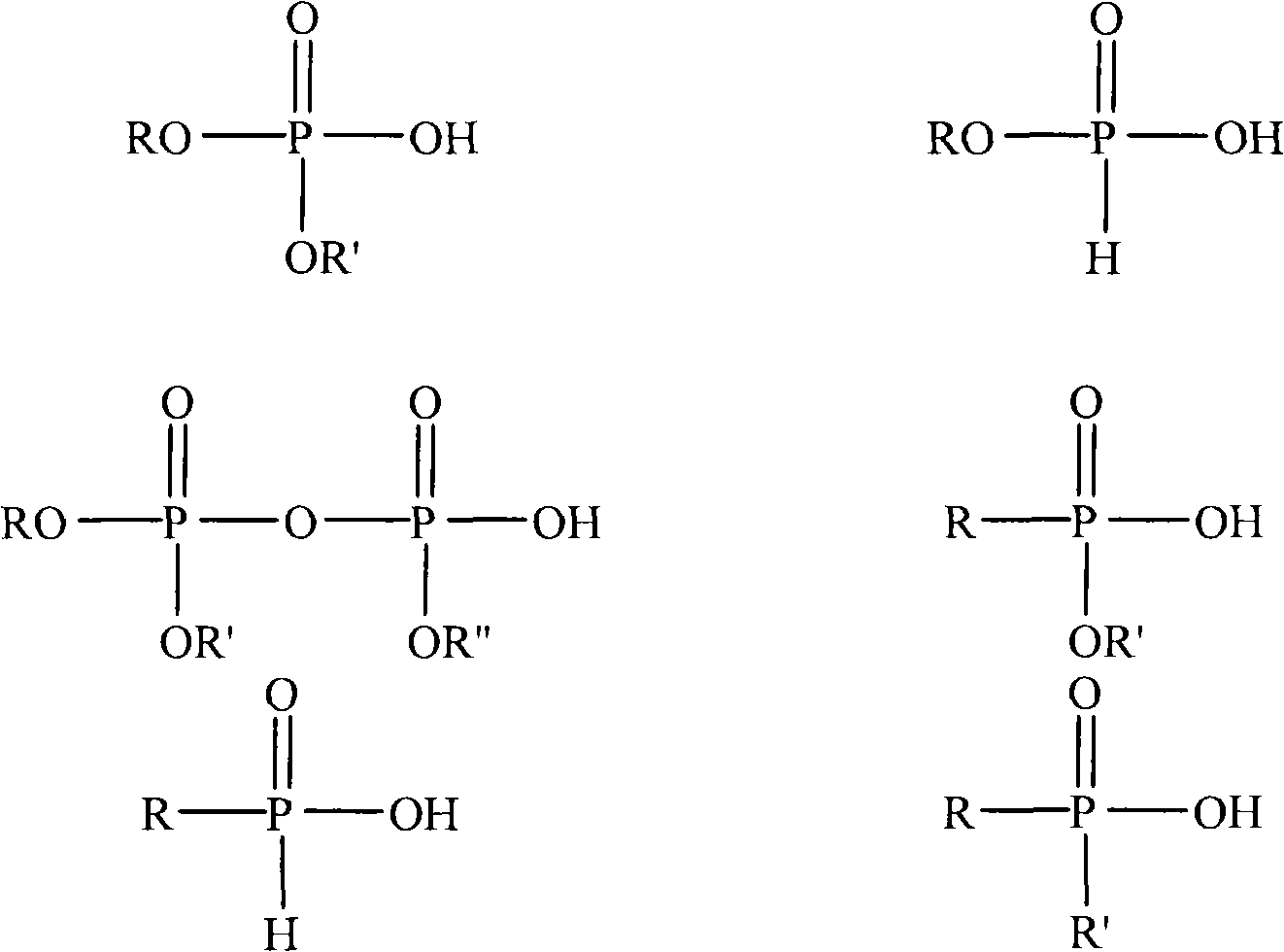
Method of making polymeric bead from phosphorous acid containing monomers
A technology of polymer beads and acid monomers, which is applied in the field of preparing polymer beads from monomers containing phosphorus-containing acid, and can solve the problems of small average particle size of polymer beads and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 2-liter laboratory reactor, 390 grams of deionized water, 138 grams of NaCl, and 2.6 grams of sodium carboxymethylcellulose were added under stirring at 200 rpm. In a monomer preparation tank, add 150 grams of glacial methacrylic acid (GMAA), 150 grams of PEM, 24 grams of divinylbenzene (63%) (DVB), and 5.8 grams of bis(4-tert-butyl-cyclohexyl)peroxide Oxydicarbonate. Stirring was stopped in the polymerization reactor, and the monomer mixture was added to the reactor. The reactor was then stirred at 150 rpm during the run. The temperature was designed to be held at room temperature for 30 minutes, heated to 58°C and held for 5 hours, then heated to 97°C and held for 3 hours. The reaction was then cooled to room temperature. The batch was washed with excess water, sieved, dried with suction on a Buchner funnel and packaged.
Embodiment 2
[0027] In a 2-liter laboratory reactor, 390 grams of deionized water, 138 grams of NaCl, and 2.6 grams of sodium carboxymethylcellulose were added under stirring at 200 rpm. In a monomer preparation tank, add 150 grams of glacial methacrylic acid (GMAA), 150 grams of PEM, 24 grams of divinylbenzene (63%) (DVB), and 5.8 grams of bis(4-tert-butyl-cyclohexyl)peroxide Oxydicarbonate. Stirring was stopped in the polymerization reactor, and the monomer mixture was added to the reactor. The reactor was then stirred at 250 rpm for 30 minutes, then heated to 58°C for 5 hours, then to 97°C for 3 hours. The reaction was then cooled to room temperature. The batch was washed with excess water, sieved, dried with suction on a Buchner funnel and packaged.
Embodiment 3
[0029] In a 2-liter laboratory reactor, 390 grams of deionized water, 138 grams of NaCl, and 2.6 grams of sodium carboxymethylcellulose were added under stirring at 200 rpm. In a monomer preparation tank, add 50 grams of glacial methacrylic acid (GMAA), 250 grams of PEM, 12 grams of divinylbenzene (63%) (DVB), and 5.8 grams of bis(4-tert-butyl-cyclohexyl)peroxide Oxydicarbonate. Stirring was stopped in the polymerization reactor, and the monomer mixture was added to the reactor. The reactor was then stirred at 150 rpm for 30 minutes, then heated to 58°C for 5 hours, then to 97°C for 3 hours. The reaction was then cooled to room temperature. The batch was washed with excess water, sieved, dried with suction on a Buchner funnel and packaged.
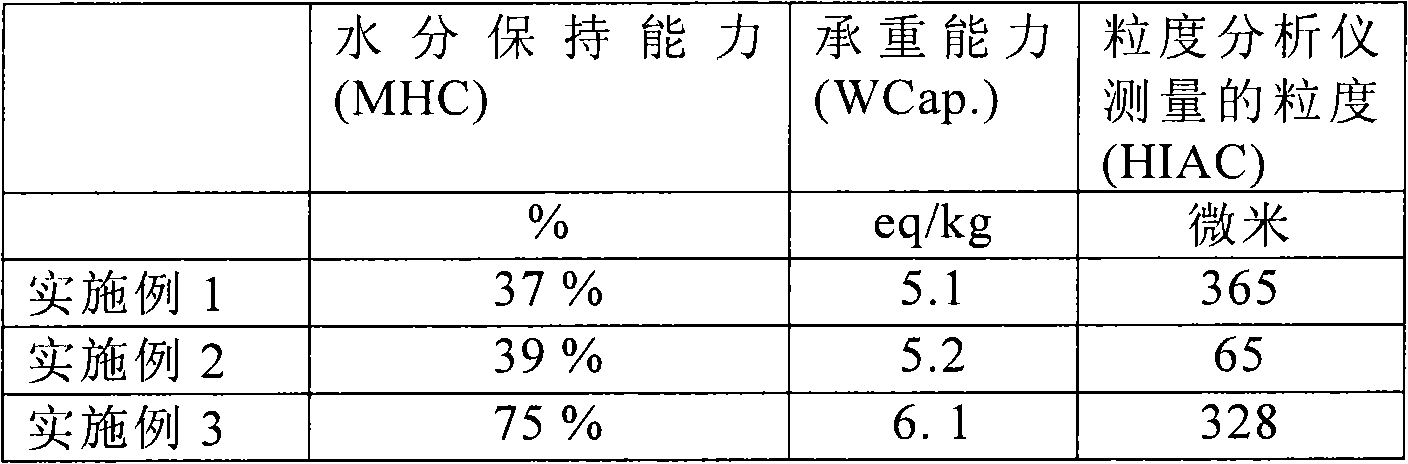
[0030] The data for Examples 1-3 are presented in the table below.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com