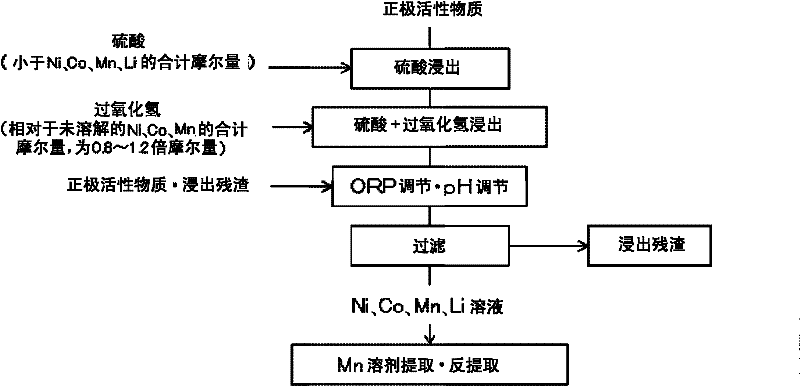
Method for leaching positive electrode active material
A positive electrode active material and leaching technology, applied in the field of leaching of valuable metals, can solve problems such as deterioration, extraction solvent oxidation, etc., and achieve the effect of reducing usage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (Example 1: For the effect of adding hydrogen peroxide after leaching with sulfuric acid)
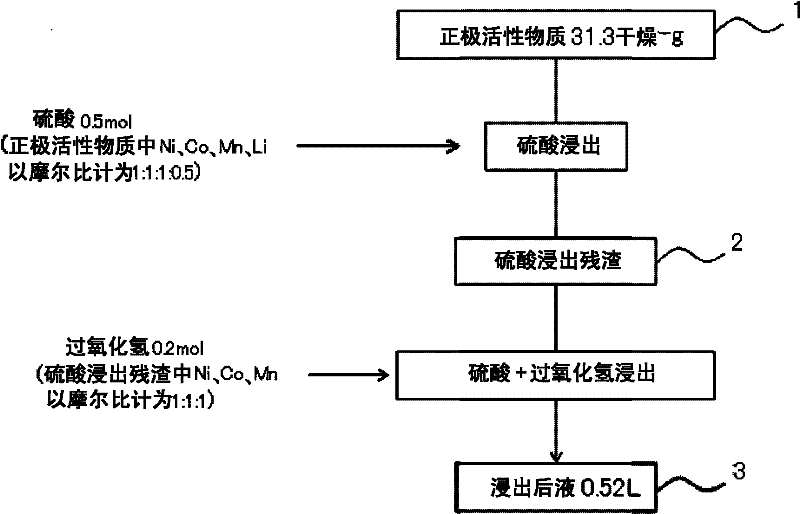
[0052] Such as figure 2 As shown in , positive electrode active material 1 (31.3 dry-g) was added to 0.5 L of 1 mol / L sulfuric acid solution (0.5 mol of sulfuric acid), and stirred at 600 rpm without heating. The amount of sulfuric acid to be added was the total amount of sulfuric acid having an equimolar amount to nickel, cobalt, and manganese in the positive electrode active material and sulfuric acid having a molar ratio of 0.5 to lithium. Table 1 shows the composition of the positive electrode active material added. The liquid temperature is 20-30°C. In addition, according to Table 2 showing the composition of the sulfuric acid leaching residue 2, by leaching using only sulfuric acid, 37 wt% of nickel, cobalt, and manganese contained in the positive electrode active material before leaching were leached, and 66 wt% of lithium was leached.
[0053] After the leaching using...
Embodiment 2
[0060] (Example 2: Manganese solvent extraction of leachate after addition of positive electrode active material and addition)
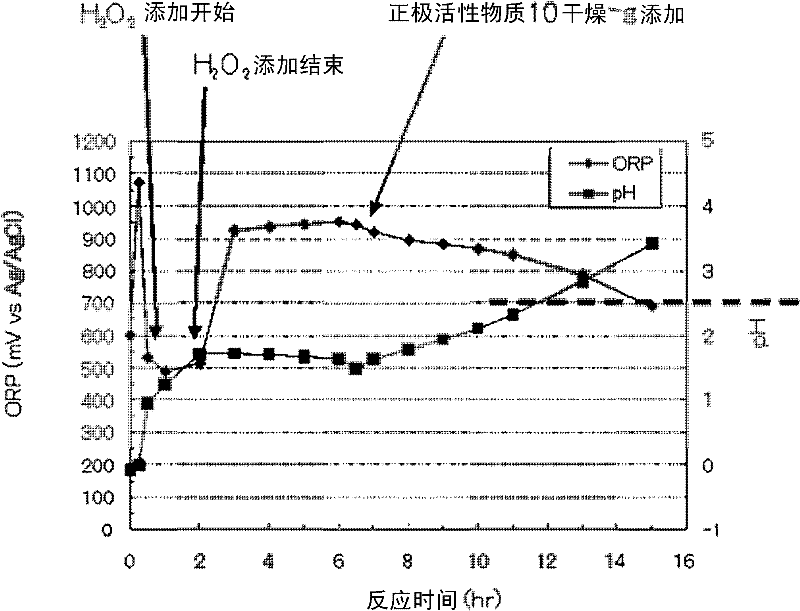
[0061] In 0.5L of the solution after leaching with sulfuric acid and hydrogen peroxide, add the positive electrode active material (10 dry-g), and stir at 600rpm at normal temperature (20-30°C), the oxidation-reduction potential ORP of the solution decreases to below 700mV (vs.Ag / AgCl). Furthermore, the pH of the solution rose above 3. show the result in image 3 . The composition of the positive electrode active material added was the same as that shown in Table 1. The time at which the positive electrode active material was added was 6.5 hours of the reaction time in the figure. Then, stirring was continued, and a decrease in the oxidation-reduction potential ORP and an increase in pH were observed. After adding the positive electrode active material, the ORP of the leached solution was 700 mV or less 8.5 hours later (15 hours after the start ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com