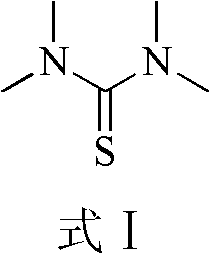
Method for preparing 1,1,3,3-tetramethyl thiourea
A technology of tetramethylthiourea and dimethylformamide, applied in the first field, can solve the problems of high raw material cost, toxicity, safety hazards, etc., and achieve the effect of low cost and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0008] Embodiment 1: 1,1,3, the preparation method of 3-tetramethylthiourea, carry out following steps successively:
[0009] Add 3.2g (0.1mol) of sulfur and 30g (about 0.4mol) of DMF to the reactor, heat until the sulfur is completely dissolved, add 3.5g (about 0.15mol) of sodium, and heat up to 120°C for 2 hours to complete the reaction. The reaction liquid was distilled, and fractions of components at 243-246°C were collected to obtain 8.5 g of 1,1,3,3-tetramethylthiourea (purity: 99%), with a yield of 64%.
Embodiment 2~5
[0011] 1,1,3, the preparation method of 3-tetramethylthiourea, change the following reaction conditions among the embodiment 1: DMF consumption (abbreviation R1), sodium consumption (abbreviation R2), reaction time (abbreviation t), temperature of reaction (abbreviation T), thereby correspondingly obtaining embodiment 2~5,1,1,3, the yield of 3-tetramethylthiourea is Y and purity etc. and specific content and data result are shown in Table 1.
[0012] Table 1
[0013] Example 2 3 4 5 R1, g 25 30 25 20 R2, g 3 3.5 2.5 3 t, h 4 5 3 3.5 T,℃ 120 140 110 130 Y,% 68 79 61 72 purity,% 99 99 99 99
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com