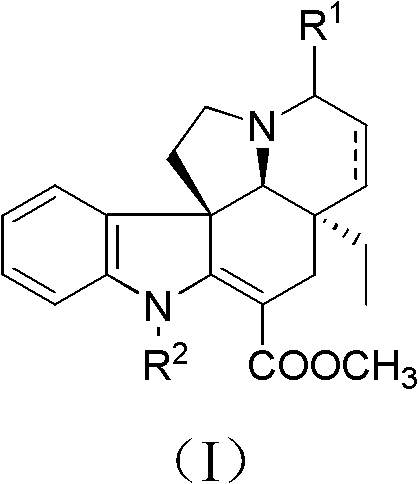
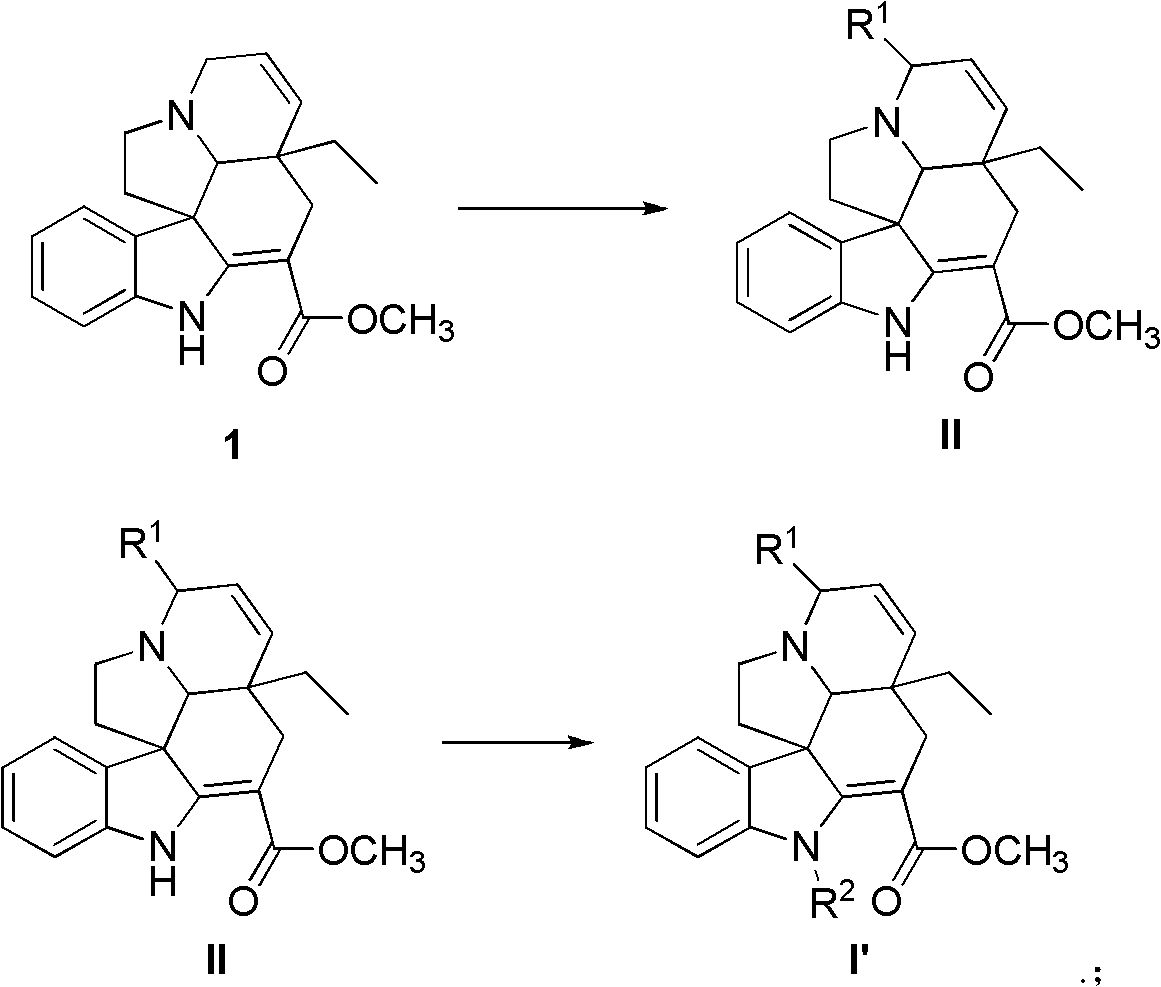
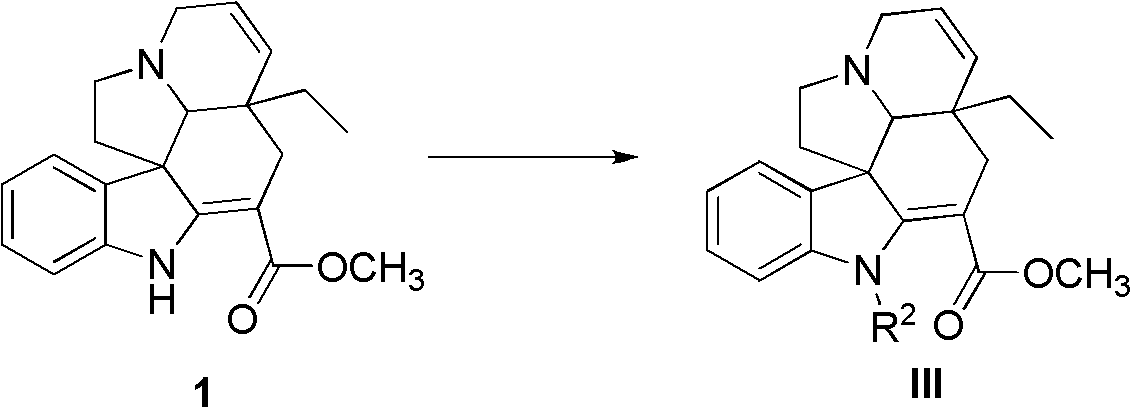
Tabersonine derivative, pharmaceutical compositions, preparing method and medical application thereof
A technology of glycyrrhizin and compound, applied in the field of application in the preparation of medicines for treating or preventing cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Preparation of methoxyglycyrrhizine and acetonylglycyrrhizine:
[0114] Glycyrrhizine hydrochloride, peroxybenzoic anhydride, NBS and 5eq methanol were dissolved in CCl4, and refluxed for 5h under anhydrous and anaerobic conditions to obtain glycyrrhizine hydrochloride methoxy derivatives, and then methoxy derivatives, trifluoromethanesulfonic acid Dissolve silver and methyl ketone in acetonitrile and react at room temperature for 10 hours to obtain acetone derivatives. ESI: 393[M+1] + , 1 H-NMR (CDCl3, 500MHz) δ: 7.34 (m, H-12), 7.13 (m, H-10), 7.02 (t, H-11), 6.86 (dd, H-9), 5.86 (m, H-14), 5.72(m, H-15), 4.04(m, H-3), 3.68(-OCH3), 3.30(d, -CH2-), 3.01-2.78(m, H-5), 2.61 (s, H-21), 2.58-2.04 (dddd, H-17), 2.16 (COCH3), 2.01 (m, H-6), 0.91 (m, H-19), 0.67 (m, H-18) .
[0115]
Embodiment 2
[0117] Alternative method for preparing acetonyl hydroglycyrrhizine:
[0118] Dissolve AIBN, NBS, 5eq acetone, and potassium carbonate or cesium carbonate as base in dichloromethane, and reflux under anhydrous and oxygen-free conditions to obtain the target compound. ESI: 393[M+1] + .
[0119] (1)
[0120]
Embodiment 3
[0122] Preparation of bromoglycyrrhizine and conversion to acetonylglycyrrhizine:
[0123] Glycyrrhizine, NBS, peroxybenzoic anhydride, in CCl 4 Reflux overnight to obtain brominated derivatives, and then catalyzed by trifluoroacetic acid, silver trifluoromethanesulfonate as base, brominated derivatives and acetone were dissolved in dichloromethane to obtain the target compound. Bromo derivatives: ESI: 415[M+1] + . Acetone derivatives: ESI: 393[M+1] + .
[0124]
[0125] The following derivatives of hydroglycyrrhizine can be prepared according to a similar method:
[0126] Acetophenonyl glycyrrhizine:
[0127]
[0128] Ethynyl hydroglycyrrhizine:
[0129]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com