Refining method of methyhaaltrexone bromide
A technology of bromnaltrexone and a purification method, which is applied in the field of chemical drug synthesis, can solve the problems such as the inability of the bromnaltrexone product to meet the purity, and achieves the effects of mild conditions, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
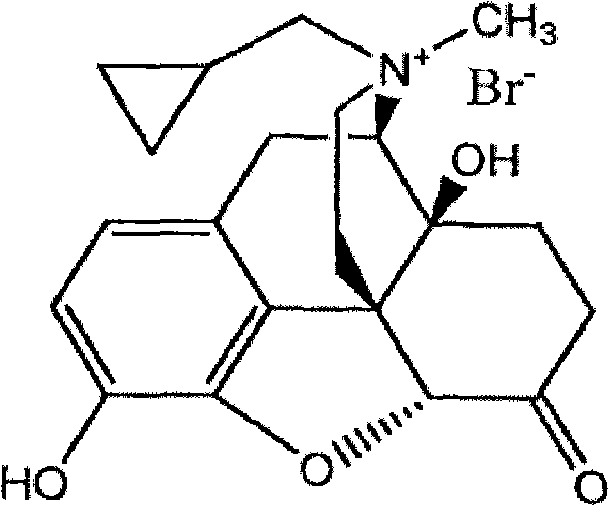
[0036] Preparation Example 1: Synthesis of Methylnaltrexone Bromide
[0037] Under the protection of nitrogen, add 11.6g (34.0mmol) naltrexone base, 175mL anhydrous acetone, 23.2mL DMF, 11.4mL (208mmol) methyl bromide into the three-necked flask, turn off the nitrogen, and react in a sealed manner at 25°C for 21 days. After the reaction is completed, reduce the pressure Concentrate the reaction solution to dryness, add 150 mL of acetone to wash, then suction filter, take the filter cake and dry it in vacuum at 80°C for 6 hours to obtain 13.4 g of crude methylnaltrexone bromide, with a yield of 90.17%.
Embodiment 1
[0038] Embodiment 1: the refining of methylnaltrexone bromide
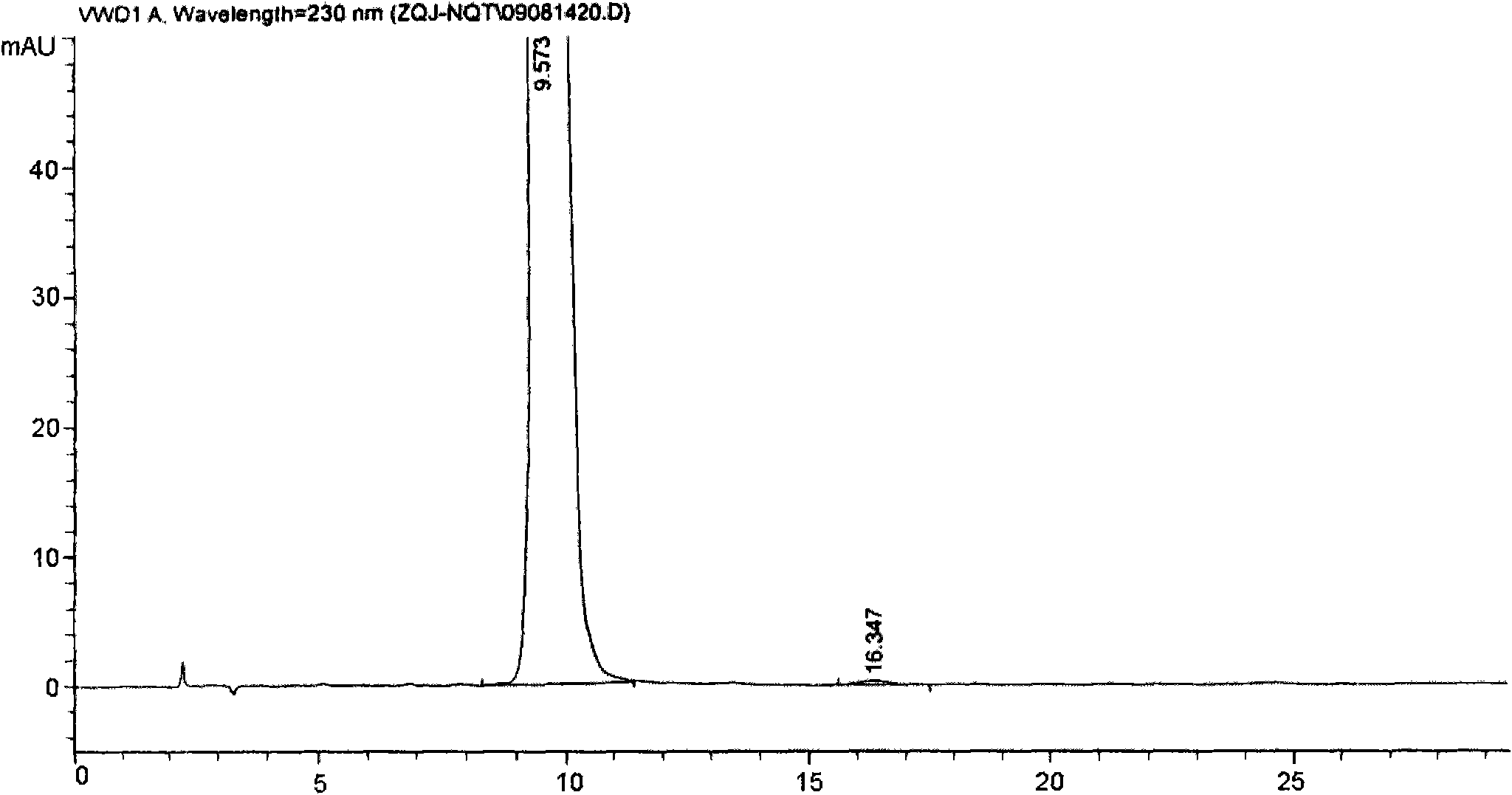
[0039] Add 13.4g of crude methylnaltrexone bromide, 75mL of methanol, and 19mL of pure water into a 250mL four-neck flask, stir and heat to dissolve, add 0.7g of activated carbon for needles after cooling slightly, reflux for 30min, cool to room temperature and stir for 4h, filter, and use for filter cake After washing with methanol and drying, vacuum drying at 80° C. for 6 hours, 10.4 g of a white product (referred to as “brommethylnaltrexone refined product 1”) was obtained, with a yield of 77.61%.
[0040]Above-mentioned 10.4g methylnaltrexone bromide refined product 1 was dissolved with appropriate amount of water and passed through reverse phase silica gel column, first eluted with 1% aqueous methanol solution, then eluted with 5% aqueous methanol solution, and monitored by thin layer chromatography (TLC). When the main component spots appeared in the layer chromatogram, the corresponding eluent was collected...
Embodiment 2
[0042] Embodiment 2: the refining of methylnaltrexone bromide
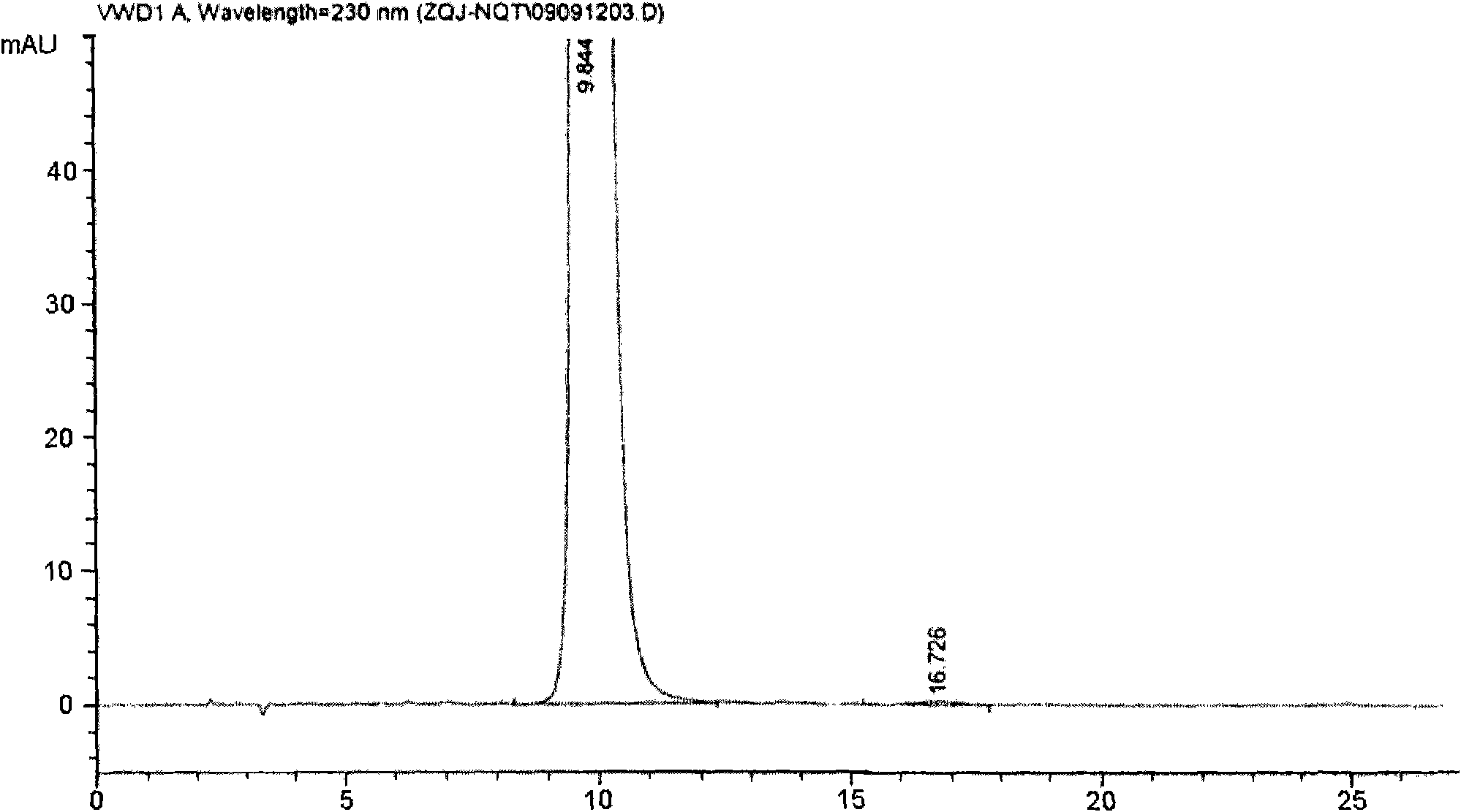
[0043] Add 13.4g of crude methylnaltrexone bromide, 75mL of methanol, and 19mL of pure water into a 250mL four-neck flask, stir and heat to dissolve, add 0.7g of activated carbon for needles after cooling slightly, reflux for 30min, cool to room temperature and stir for 4h. After filtering, the filter cake was washed with methanol and sucked dry, and dried in vacuum at 80° C. for 6 hours to obtain 10.2 g of a white product (referred to as “brommethylnaltrexone refined product 1”).
[0044] Above-mentioned 10.2g methylnaltrexone bromide refined product 1 is dissolved with appropriate amount of water and passes through reverse phase silica gel column, first with 1% aqueous methanol elution, then with 5% methanol aqueous elution, thin-layer chromatography (TLC) monitoring, when thin When the main component spots appeared in the layer chromatogram, the corresponding eluent was collected, and the collected eluent was c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com