Ursolic acid derivative and preparation method thereof
A technology of ursolic acid and derivatives, applied in the field of derivatives of ursolic acid, can solve the problems of unsatisfactory resistance to other tumors, complicated preparation methods and high industrial production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
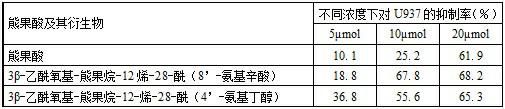
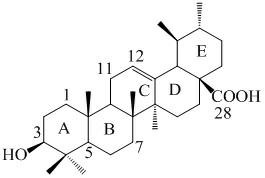
[0025] One, a kind of ursolic acid derivative, the 3-position of ursolic acid parent body is 3β-acetoxyl group in this derivative structure, in the present invention, is 28-acyl (8 ' in the 28-position of ursolic acid parent body) -amino caprylic acid), the ursolic acid derivative is 3β-acetoxy-arbutane-12-ene-28-acyl (8'-amino caprylic acid), and its structural formula I is:
[0026] ;
[0027] Alternatively, at the 28-position of the parent ursolic acid is 28-acyl (4'-aminobutanol), the ursolic acid derivative is 3β-acetoxy-arbutane-12-en-28-yl (4 '-aminobutanol), its structural formula II is:
[0028] .
[0029] Two, a kind of preparation method of ursolic acid derivative, what this method prepared is the ursolic acid derivative described in this specific embodiment one; The method has following preparation steps:
[0030] (1) Reaction of ursolic acid and acetic anhydride to acetylate and protect the 3-hydroxyl of ursolic acid to generate 3-O-acetyl ursolic acid;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com