Cysteine for physiological injection
A cysteine, physiological technology, applied in the field of cysteine for physiological injection, can solve problems such as easy oxidation or dimerization, instability of cysteine solution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0285] Embodiment 1: the production of physiological cysteine solution
[0286] This example illustrates the desired composition, pH range and storage conditions of a physiological solution of cysteine.
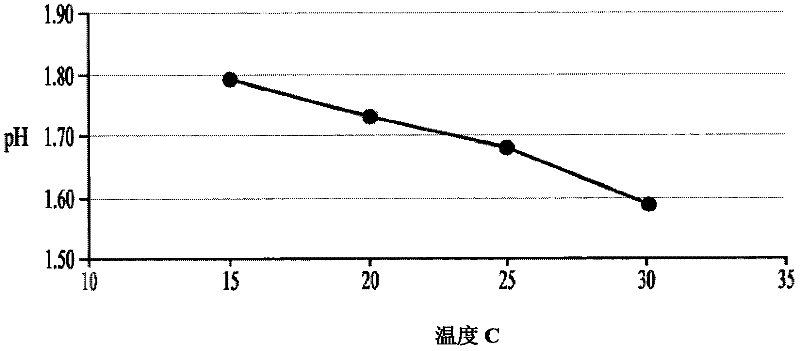
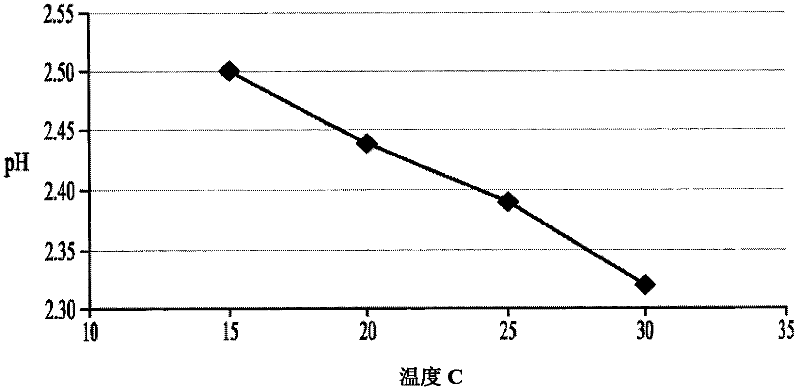
[0287] To develop stable cysteine formulations for physiological administration, several factors need to be considered. First, cysteine hydrochloride solutions at concentrations of 50 mg / ml or above in 0.9% saline have a very low natural pH - about pH 0.8 to pH 1.0. Second, administering a cysteine solution with a pH value of 4.0-5.0 can avoid vascular irritation or damage caused by cysteine administration. Third, the basic form of cysteine is insoluble at concentrations above 50 mg / ml and is generally considered too dilute for convenient administration to patients. In contrast, solutions containing 150-200 mg / ml or more cysteine are more convenient for intravenous administration.
[0288] This example illustrates which types of cysteine formulations can be...
Embodiment 2
[0344] Example 2: Cysteine Precipitation When Solutions Are Exposed to Air
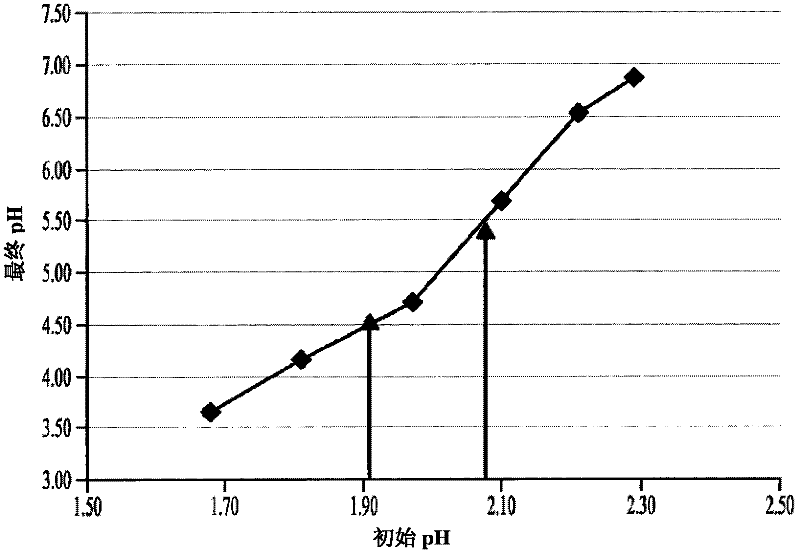
[0345] This example shows that exposing a low pH cysteine solution to air during neutralization will result in the precipitation of cysteine. In this example, the formation of particulate matter was detected after the L-cysteine hydrochloride monohydrate preparation was mixed with Tris neutralizing reagent.
[0346] Materials and methods
[0347] The following materials and equipment were used:
[0348] 1. Tris, 99.9%, ultra-pure grade, batch number 17120CH
[0349] 2. L-cysteine hydrochloride monohydrate, Sigma Aldrich, R&D production grade, lot number 087K0707611
[0350] 3. Anhydrous sodium acetate, JT Baker, ACS grade, lot number B31151
[0351] 4. L-Ascorbic acid, Sigma Aldrich, ultra-pure grade, lot number 106K0053
[0352] 5. Calcium disodium salt of EDTA, hydrate, 98% purity, Aldrich lot number 01929KH
[0353] 6. Glacial acetic acid, Mallinckrodt, USP grade, lot number E40005
...
Embodiment 3
[0411] Example 3: Preliminary Cysteine Toxicological Study
[0412] This example describes toxicology studies conducted in accordance with FDA guidelines.
[0413] Toxicological tests of L-cysteine were performed in rats, dogs and monkeys. Monkeys have been used routinely for several years and monkeys have been used for several years to evaluate the effects of different neuromuscular blocking agents and reversal of neuromuscular blockade with different doses of cysteine. Give conscious rats 750mg / kg L-cysteine by intravenous injection, and give conscious dogs 400mg / kg by intravenous injection. The neuromuscular blocking agent CW002, which is the R-trans, R-trans isomer of the compound shown below, was administered intravenously to anesthetized dogs at 200 mg / kg.
[0414]
[0415] Anesthetized dogs were awakened and sacrificed 2 days or 2 weeks later.
[0416] No animals exhibited clinical, biochemical, or histological evidence of organ toxicity. No toxic effects w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com