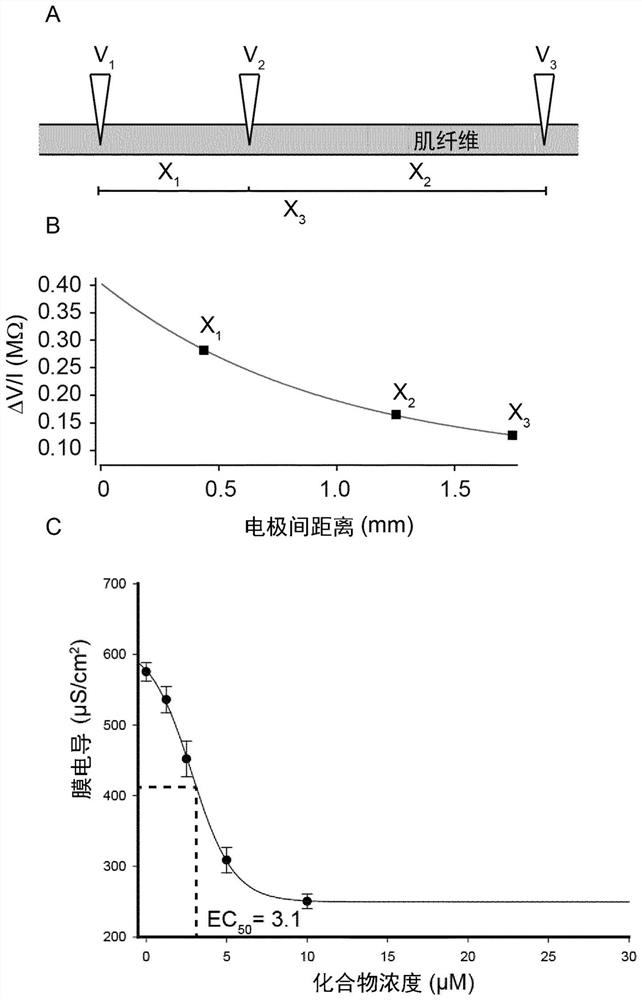
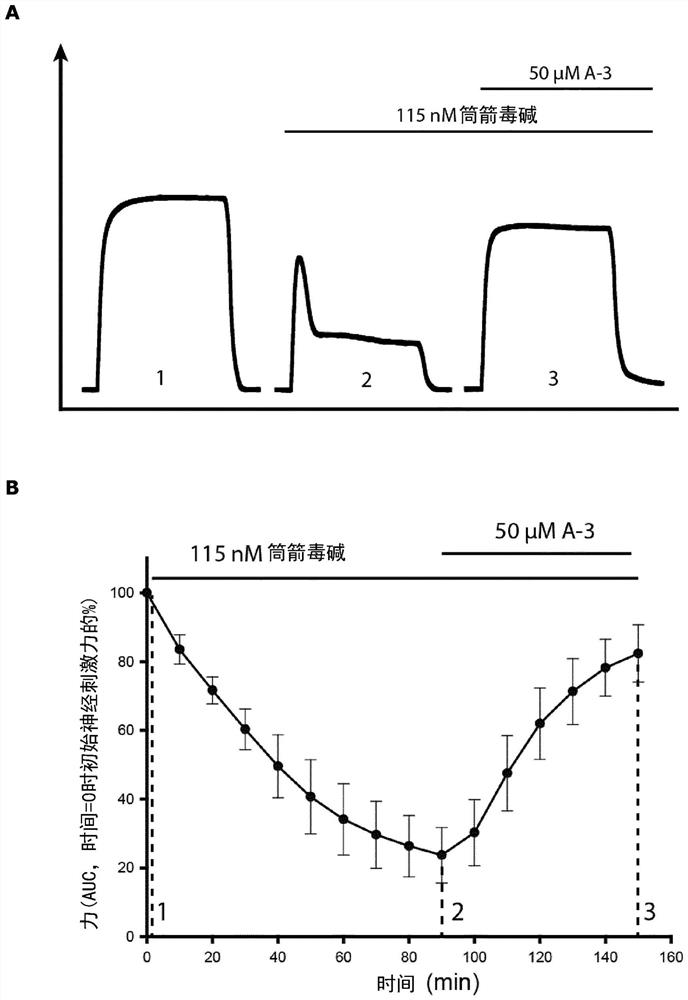
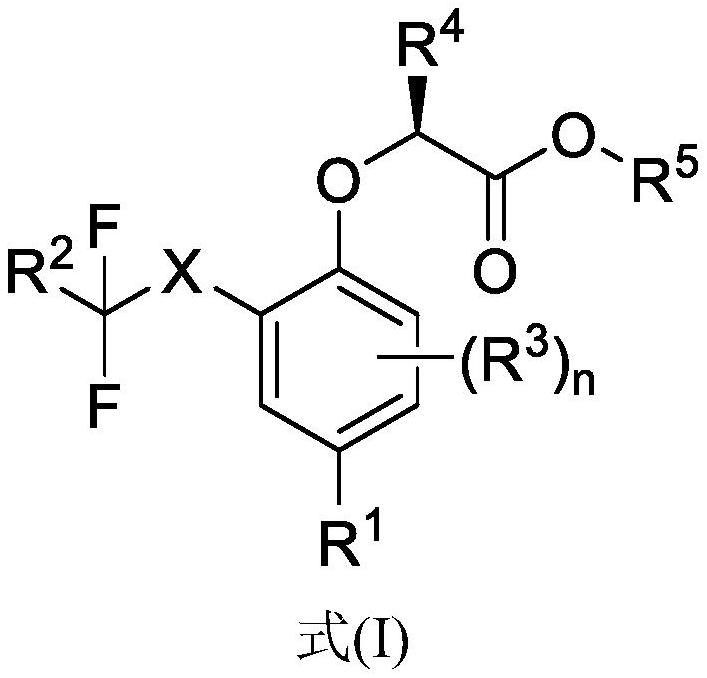
Compounds for the treatment of neuromuscular disorders
A technology of compounds, solvates, in the field of compounds for the treatment of neuromuscular disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0825] Example 1: Synthesis of (2S)-2-[4-bromo-2-(1,1-difluoroethyl)phenoxy]propionic acid
[0826]
[0827] Step 1: Synthesis of (S)-methyl 2-(2-acetyl-4-bromophenoxy)propionate 2
[0828] Under stirring, to (R)-methyl 2-hydroxypropionate 1 (3.07g, 29.44mmol), 1-(5-bromo-2-hydroxyphenyl)ethanone (6.33g, 29.44 mmol) and TPP (9.28 g, 35.38 mmol) in 150 mL of DCM was slowly added DIAD (6.96 mL). The ice bath was then removed, and the mixture was stirred at room temperature overnight, then concentrated under reduced pressure. After removing the solvent, the residue was passed through a short silica gel column (Hexane-EtOAc, 25:1, 10:1) to give the desired product 2 (7.9 g, 89%) as a pale yellow oil.
[0829] 1 H NMR (300MHz, CDCl 3 )δ7.83(d,1H); 7.48(dd,1H); 6.67(d,1H); 4.87(q,1H); 3.75(s,3H); 2.67(s,3H); 1.68(d,3H ).
[0830] Step 2: Synthesis of (S)-methyl 2-(4-bromo-2-(1,1-difluoroethyl)phenoxy)propionate 3
[0831] To (S)-methyl 2-(2-acetyl-4-bromophenoxy)propio...
Embodiment 2
[0838] Example 2: Synthesis of (S)-2-(4-bromo-2-(1,1-difluoropropyl)phenoxy)propionic acid
[0839] This compound was prepared as described in Example 1.
[0840] (S)-Methyl 2-(4-bromo-2-propionylphenoxy)propionate
[0841] 1H NMR (300MHz, CDCl3) δ7.84(d,1H),7.52(dd,1H),6.72(d,1H),4.91(q,1H),3.82(s,3H),3.16–3.07(m, 2H), 1.73(d,3H), 1.23(t,3H).
[0842] (S)-Methyl 2-(4-bromo-2-(1,1-difluoropropyl)phenoxy)propionate
[0843] 1H NMR (300MHz, CDCl3) δ7.48(dd,1H),7.27(dd,1H),6.50(d,1H),4.63(q,1H),3.59(s,3H),3.34–3.14(m, 2H), 1.48(d, 3H), 0.79(t, 3H). 19 F NMR (300 MHz, CDCl3) δ -93.5 and -99 ppm.
[0844] (S)-2-(4-Bromo-2-(1,1-difluoropropyl)phenoxy)propionic acid
[0845] 1H NMR (300MHz, CDCl3) δ9.69(br s,1H),7.45(dd,1H),7.27(dd,1H),6.52(d,1H),4.63(q,1H),2.9–2.05(m ,2H), 1.50(d,3H), 0.74(t,3H). 19 F NMR (300MHz, CDCl3) δ-94 and -98ppm.
[0846] MS(ES-): m / z 321.2(M-H) - . HPLC retention time: 13.56min
[0847] Chiral SCF method 1: 1.09 min (>97.2% e.e.). (R)-ena...
Embodiment 3
[0848] Example 3: Synthesis of (R)-2-(4-bromo-2-(1,1-difluoropropyl)phenoxy)-3-fluoropropionic acid
[0849]
[0850] Synthesis of (S)-1-(benzyloxy)-3-fluoropropan-2-ol
[0851] (R)-benzyl glycidyl ether (200g, 1.22mol, 1.0eq.), CsF (191g, 1.26mol, 1.0eq.) and KHF 2 (99g, 1.27mol, 1.0eq.) at RT and N 2 Add to the flask. Add 1M TBAF in THF (3.91 L, 3.2 eq.) [no exotherm], and react at RT and N 2 Stir down. The reaction was heated to 65 °C over 1 h 30 min and stirred at 65 °C for 21 h at which time LC showed 76% product. Cool the reaction to 25 °C and add KHF 2 (9.9 g, 0.13 mol, 0.1 eq) and CsF (19.1 g, 0.13 mol, 0.1 eq). The reaction was heated to 65°C for 18 hours at which time LC showed 77% product. The reaction was cooled to room temperature and H was added over 5 min 2 O (3.90 L) [mildly exothermic]. The aqueous phase was extracted with toluene (3x1.00L). The combined organic extracts were washed with 20% K 2 CO 3 (aq)(1.90L) and H 2 O (2 x 1.90 L) washes ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com