Application of VEGF (Vascular Endothelial Growth Factor) receptor fusion protein in preparation of medicament treating sepsis
A technology of fusion protein and therapeutic drug, applied in the field of VEGF inhibitor, which can solve problems such as sepsis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Fusion protein FP1, FP2, FP3, FP4, FP5, FP6, FP7, FP8 and VEGF binding affinity experiments
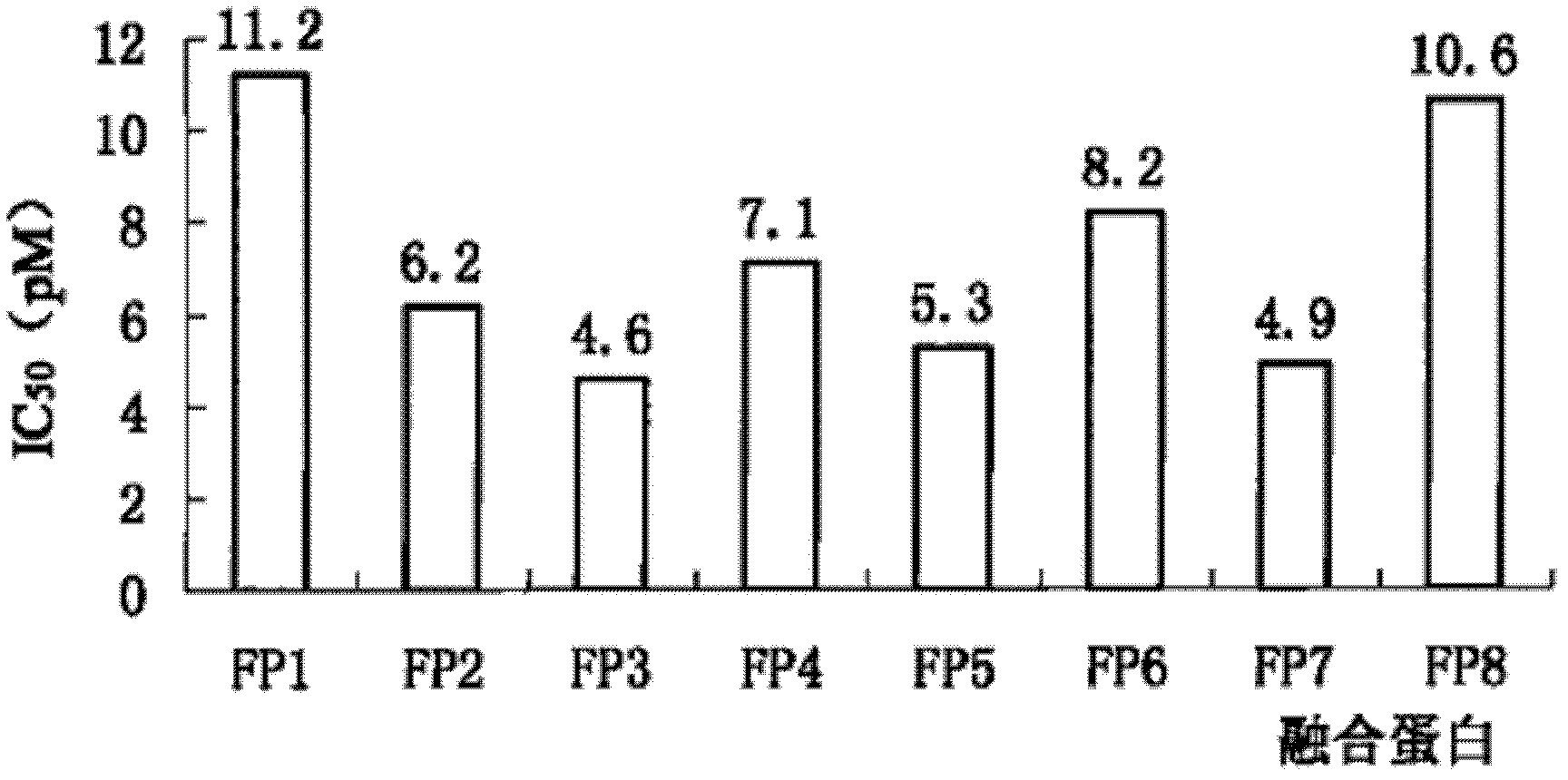
[0043] The present invention determines the ability of various fusion proteins to bind VEGF by measuring the amount of free VEGF. In this experiment, a certain amount of VEGF (10PM) was added to a centrifuge tube, and then fusion proteins FP1, FP2, FP3, FP4, FP5, FP6, FP7, and FP8 containing a certain concentration gradient were added to centrifuge tubes containing VEGF. tube, mix well and store in a 37°C incubator for one hour. One hour later, the free VEGF in each centrifuge tube was determined by the VEGF detection kit (VEGF assay Kit) provided by R&D systems (R&D systems). The Half Maximal Inhibitory Concentration (IC50) was used as the indicator of affinity, and the smaller the IC50, the stronger the affinity.
[0044]The fusion proteins FP1, FP2, FP3, FP4, FP5, FP6, FP7, and FP8 can all effectively bind to VEGF, and their IC50 values are shown in the appendi...
Embodiment 2
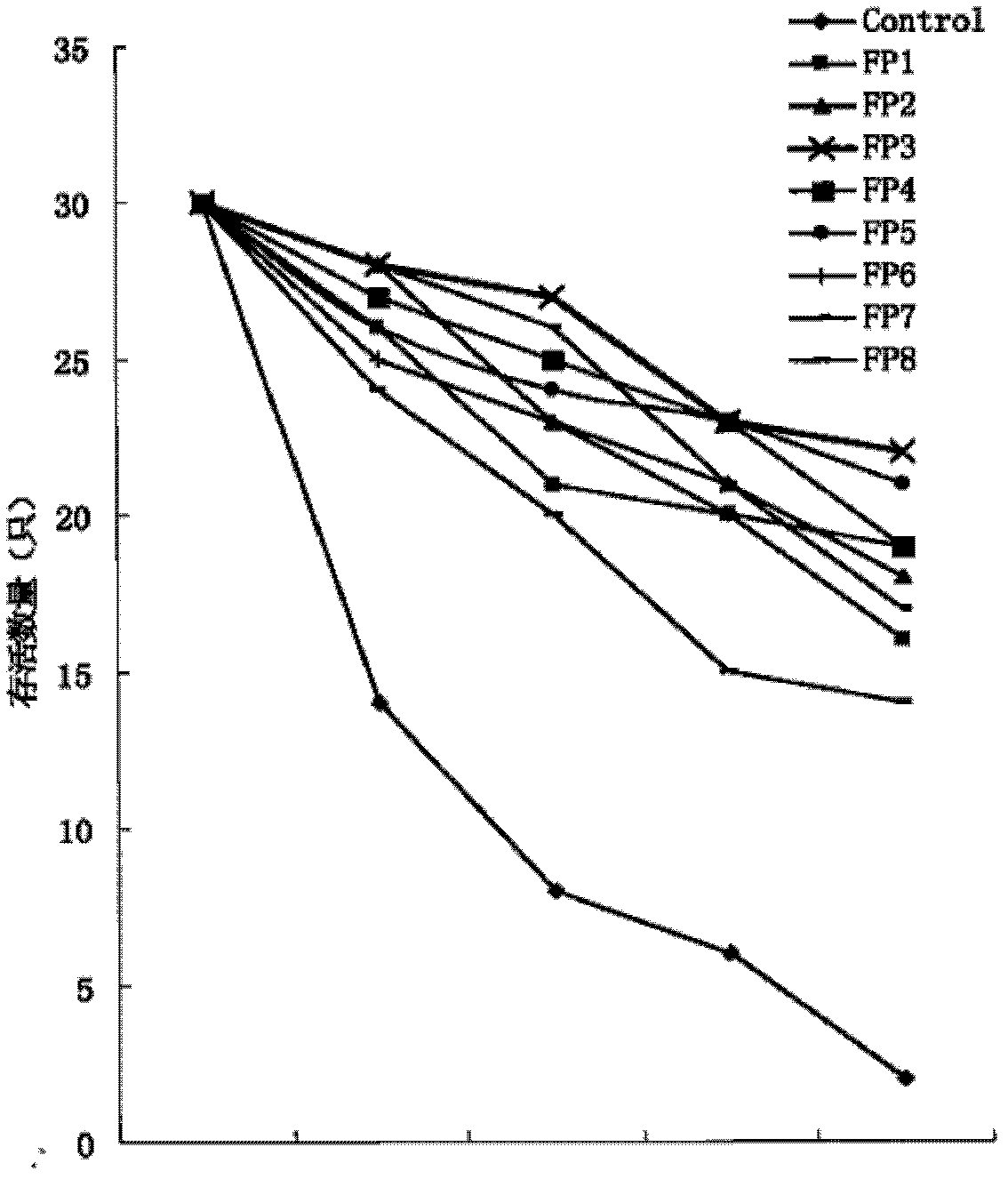
[0045] Example 2 Effects of fusion proteins FP1, FP2, FP3, FP4, FP5, FP6, FP7, FP8 on the survival rate and plasma VEGF of LPS sepsis rats
[0046] In this experiment, a rat model of sepsis was established by intravenous injection of lipopolysaccharide (LPS) (refer to Schultz M J et al. (2002), Ann Med, 34:573-581). 270 clean-grade male rats, weighing 200g-240g, were each given intravenous injection of lipopolysaccharide (LPS) 30mg / kg, and were randomly divided into 9 groups, 30 in each group. One group is the control group, and the rest are the fusion protein FP1, FP2, FP3, FP4, FP5, FP6, FP7, FP8 treatment groups in turn. The control group received an intravenous injection of equal volume of normal saline, and the fusion protein treatment group received an intravenous injection of 10 mg / kg of the corresponding fusion protein. The number of surviving rats was recorded from 0 to 96 hours after completion.
[0047] Blood was drawn from the heart of the rats that died in the e...
Embodiment 3
[0050] Example 3 Effects of fusion proteins FP1 and FP3 on the level of TNF-α in CLP sepsis rats
[0051] Clean-grade male rats, weighing 230g-260g, were used to prepare rat models of cecal ligation and puncture (CLP) sepsis (refer to Chaudry IH et al. (1979), Surgery, 85: 205-211). 112 animals were randomized into groups. 1, 8 rats in the normal group; 2, 8 rats in the sham-operated group; 3, 32 rats in the control group: executed at 2 hours, 8 hours, 24 hours, and 48 hours after operation (each 8 rats); 4, FP1 administration group 32 rats: FP16mg / kg was administered intravenously after the operation, and they were killed at 2, 8, 24, and 48 hours respectively (8 each); , 24, 48 hours to kill (8 each). The experimental animals were killed alive after anesthesia, the abdominal aortic blood was collected, the serum was separated, and stored at -80°C after aliquoting; the liver and lungs were also taken and stored in liquid nitrogen.
[0052] Detection of TNF-α: Liver and lun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com