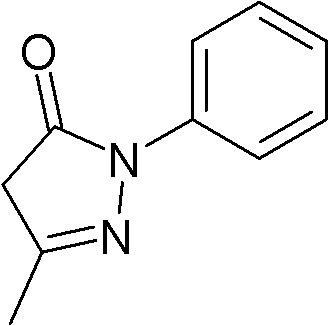
New use of 3-methyl-1-phenyl-2-pyrazoline-5-ketone
A technology of pyrazoline and phenyl, which is applied in the field of new application of 3-methyl-1-phenyl-2-pyrazolin-5-one, which can solve the problem that is difficult to cure, cannot prevent recurrence, and affects the quality of life of patients And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1 medicine preparation
[0017] Get propranolol hydrochloride 5g and take 50% ethanol as solvent, make medicine dissolve, add Azone-propylene glycol 5ml as composite accelerator, add PVPK305g to be film-forming material, add 50% ethanol to 100ml, prepare propranolol hydrochloride milk liniment.
[0018] The Edaravone injection used in the following examples is a commercially available drug; trade name: Bicun; specification: 10mg / 5mL; production unit: Nanjing Xiansheng Dongyuan Pharmaceutical Co., Ltd.
[0019] The methotrexate for injection used in the following examples is a commercially available drug; specification: 0.1 g / bottle; production unit: Jiangsu Hengrui Medicine Co., Ltd.
Embodiment 2
[0020] Example 2 Edaravone Animal Model Evaluation of Therapeutic Effect on Psoriasis
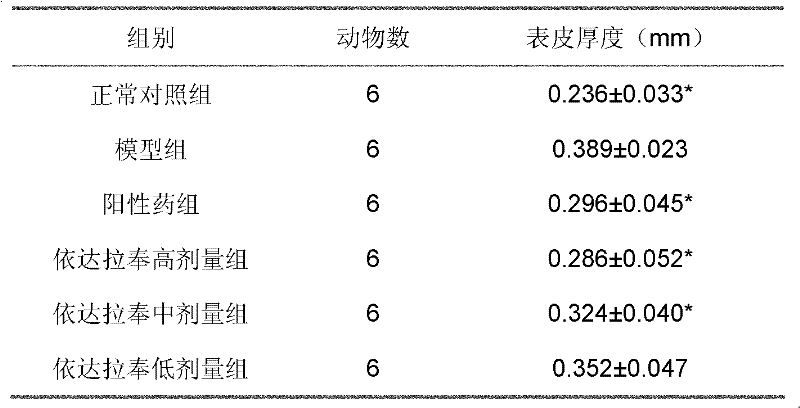
[0021] Forty-four guinea pigs with a body weight of 300-400 g, half male and half male, were randomly divided into a blank group of 10 and a model group of 34 guinea pigs. Each animal in the model group was coated with 5% propranolol hydrochloride emulsion liniment on the left auricle skin (0.3g / cm2) with a glass rod, twice a day for 2 consecutive weeks. After the last administration, 4 guinea pigs in the normal group and 4 guinea pigs in the model group were killed to take skin samples of the left auricle, fixed in formaldehyde, stained with HE, and observed with a light microscope to evaluate the quality of the model.
[0022] The remaining 30 guinea pigs in the model group were randomly divided into 5 groups, namely the model group, the positive drug control group of methotrexate (1 mg / kg), and the high, medium and low dose (6, 3, 1.5 mg / kg) groups of edaravone. The model group did not ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com