Establishment and application of hepatocellular carcinoma cell line HCC-LY10
A HCC-LY10, liver cancer cell technology, applied in the direction of tumor/cancer cells, animal cells, vertebrate cells, etc., can solve the problems of poor tumorigenicity, non-expression of markers, unsatisfactory and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Establishment of hepatocarcinoma cell line HCC-LY10
[0068] (a) source
[0069] The cell line comes from a male liver cancer patient (patient number: 604648), and its clinical information is shown in the table below.
[0070] Table 1
[0071]
[0072] (b) method
[0073] The primary liver cancer tissue of the liver cancer patient was inoculated into immunodeficient mice, and the tumor tissue was taken for repeated inoculation and passage, and finally a stable passage cell line was obtained. Specifically include the following processes:
[0074]
[0075]
[0076] The tumor-bearing NOD / SCID mice were sacrificed painlessly, and the subcutaneous tumors were aseptically extracted. Place the tumor in a pre-cooled PBS culture dish, fully clean up the tumor capsule and necrotic part of the tumor, use ophthalmic scissors to cut the tumor tissue into small pieces with a size of about 5×5×5 mm, and add a small amount of liver cells The culture medium is cultivated ...
Embodiment 2
[0081] Biological characteristics of human hepatoma cell line HCC-LY10
[0082] In this example, the biological characteristics of the purebred human liver cancer cell line HCC-LY10 with the deposit number CCTCC NO: C2010100 were detected by conventional methods. The result is as follows:
[0083] 2.1 Cell morphology:
[0084] Observation of HCC-LY10 liver cancer cells under an inverted phase-contrast microscope reveals that the cells are polygonal or round, uniform in shape, multinucleated giant cells can be seen, and the cell proliferation speed is fast, which can be passaged every 48 hours ( image 3 ).
[0085] 2.2 Enzyme / GFP double labeling
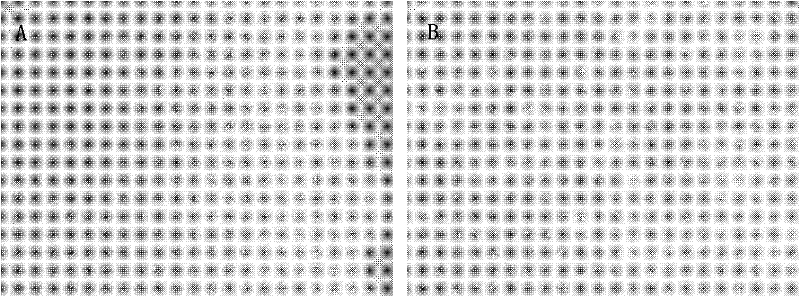
[0086] The results of luciferase / GFP double labeling of HCC-LY10 cell line are as follows Figure 4 (right panel), indicating that the cells were all labeled with green fluorescent protein (GFP).
[0087] 2.3 Karyotype analysis
[0088] By adding colchicine (final concentration: 0.04 μg / ml) to the cell culture medium, a large n...
Embodiment 3
[0115] Establishment of Animal Model of Liver Cancer Using Human Hepatoma Cell Line
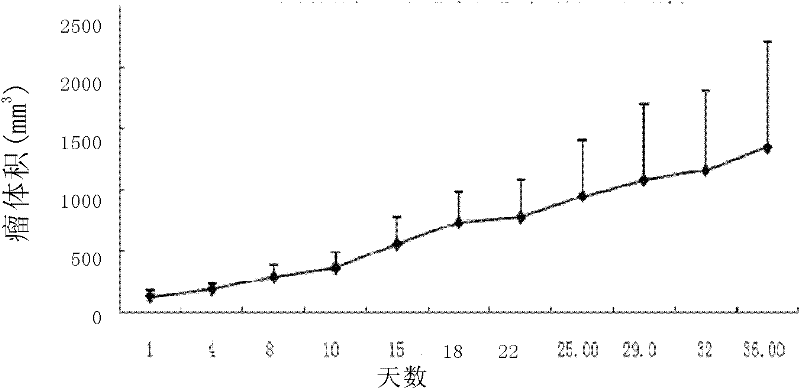
[0116] Take the human liver cancer cell line HCC-LY10 (preservation number: CCTCC C2010100) obtained in Example 1, digest it with the digestive solution (0.25% trypsin, 0.02% EDTA, pH7.3), collect and count, and count as 1×10 6 or 1×10 7 Number of cells / mouse Inoculate subcutaneously on the right chest wall of 20 Balb / c nude mice aged 4-6 weeks.
[0117] Between 14 and 60 days after inoculation, tumors appeared in all 20 mice. Pathological detection was carried out by the same method as in Example 2, and it was confirmed that hepatocellular carcinoma was induced in the animal model.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com