Method for producing 3,4-disubstituted pyrrolidine derivative and production intermediate thereof
A technology of derivatives and pyrrolidine, which is applied in the production of 3,4-disubstituted pyrrolidine derivatives and its production intermediates, and can solve the problems of reduced optical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] The preparation method of the present invention is shown in Scheme 1.
[0107] [chemical 11]
[0108] plan 1
[0109]
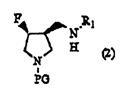
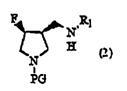
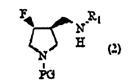
[0110] In Scheme 1, PG in formula (1) and formula (2) and R in formula (1) to formula (3) 1 has the same meaning as above.
[0111] I. Step 1
[0112] Step 1 is to fluorinate 4-hydroxyl-3-(N-substituted aminomethyl)pyrrolidine derivatives or their enantiomers shown in general formula (1) by using sulfur tetrafluoride derivatives to convert the general A step of converting the hydroxyl group of the compound represented by formula (1) or its enantiomer into a fluorine group.
[0113] In Step 1, the sulfur tetrafluoride derivative acts as a fluorinating agent. Examples of "sulfur tetrafluoride derivatives" include (dimethylamino)sulfur trifluoride (Methyl DAST), (diethylamino)sulfur trifluoride (DAST), morpholinosulfur trifluoride (Morpho-DAST ) and bis(2-methoxyethyl)aminosulfur trifluoride (Dexo-Fluor).
[0114] Among them, morpholinosulfur trif...
Embodiment 1
[0169] Benzyl (3S,4R)-3-(cyclopropylaminomethyl)-4-hydroxypyrrolidine-1-carboxylate obtained by the method described in Reference Example was dissolved in a solvent (acetonitrile, 6 times). A fluorinating agent (Deoxo-Fluor, 4 equivalents) was added at an internal temperature of -5 to 5°C, followed by stirring at an internal temperature of -5 to 5°C for 1 hour, increasing the temperature, and then internally Stirred at room temperature for 7 hours and allowed to stand overnight at room temperature (Table 1). The reaction solution was detected by HPLC. The results are listed in Table 7.
Embodiment 2
[0171] Benzyl (3S,4R)-3-(cyclopropylaminomethyl)-4-hydroxypyrrolidine-1-carboxylate obtained by the method described in Reference Example was dissolved in a solvent (acetonitrile, 6 times). Then, an additive (triethylamine pentahydrofluoric acid, 2 equivalents) was added at an internal temperature of -5 to 10°C, followed by stirring at an internal temperature of -5 to 5°C for 0.5 hours. Then, a fluorinating agent (Deoxo-Fluor) was added at an internal temperature of -5 to 5°C, followed by stirring at an internal temperature of -5 to 5°C for 1 hour, and then the temperature was raised, followed by internal Stirred at room temperature for 7 hours, then allowed to stand overnight at room temperature (Table 1). The reaction solution was detected by HPLC. The results are listed in Table 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com