Method for preparing soybean isoflavone aglycones by catalyzing hydrolysis of soybean isoflavone glycosides via citric acid
A technology of soybean isoflavones and citric acid, applied in the direction of organic chemistry, etc., to achieve the effect of simple operation and low acidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
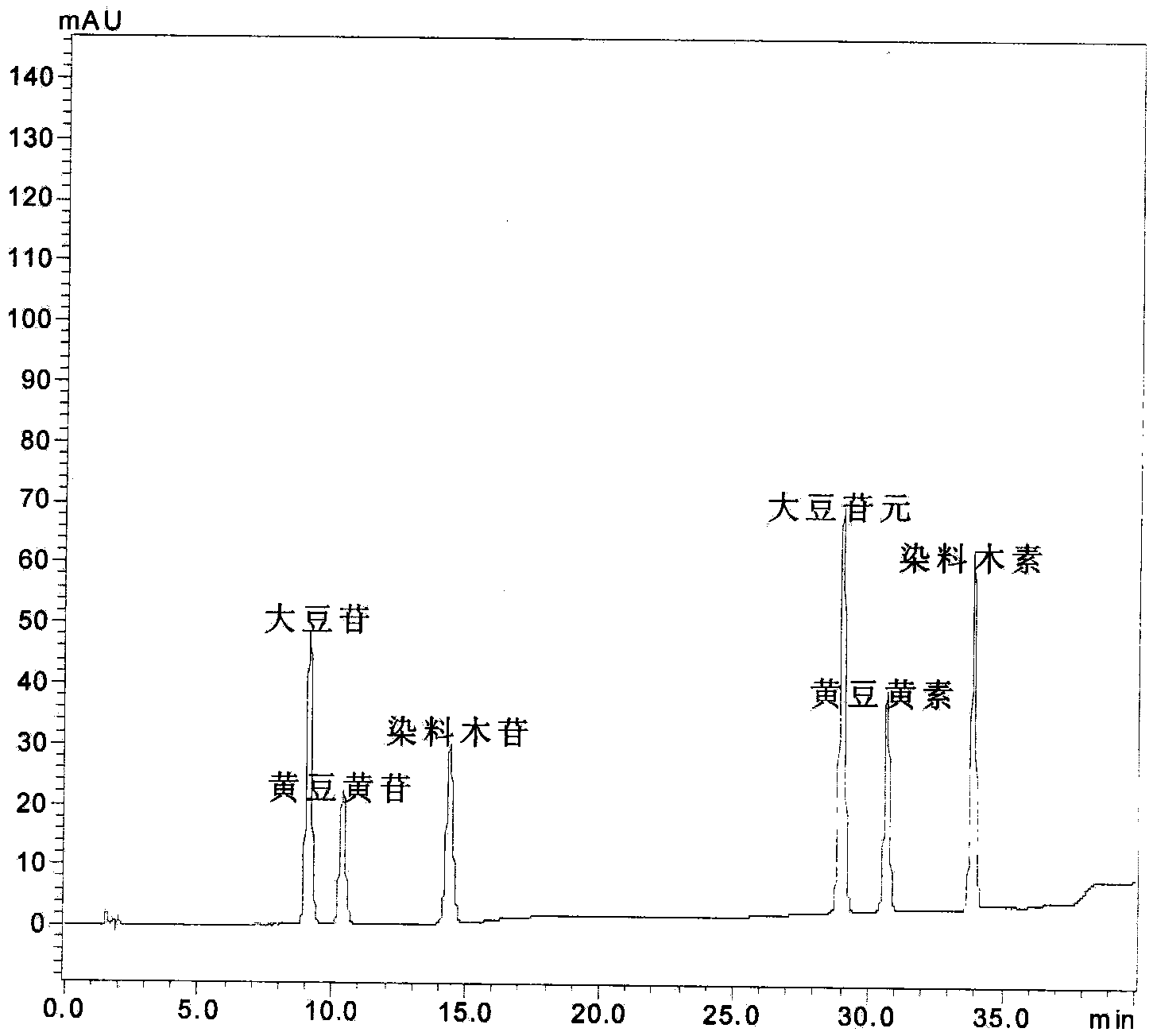
[0021] Take 50mg of 10.0% soybean isoflavone glycosides into a 50mL round bottom flask, add 2.5mol·L -1 10 mL of citric acid aqueous solution, ultrasonic water bath for 1 hour, ultrasonic frequency 45KHz, water bath temperature 75°C. The reaction solution was transferred to an autoclave, the reaction temperature was 120°C, and the reaction time was 4 hours. After the reaction was completed, the reaction solution was cooled to room temperature.
[0022] A certain amount of the reaction solution was neutralized with saturated sodium carbonate aqueous solution to pH=7, extracted with ethyl acetate, the ester layer was taken, spotted on a silica gel plate, and put in a developing solvent (ethyl acetate: chloroform: methanol = 5:5). :2) Expand in the middle, observe the degree of hydrolysis under ultraviolet light; take another certain amount of reaction solution diluted to 100ppm with methanol, and use HPLC to qualitatively and quantitatively detect the glycoside and aglycon content o...
Embodiment 2
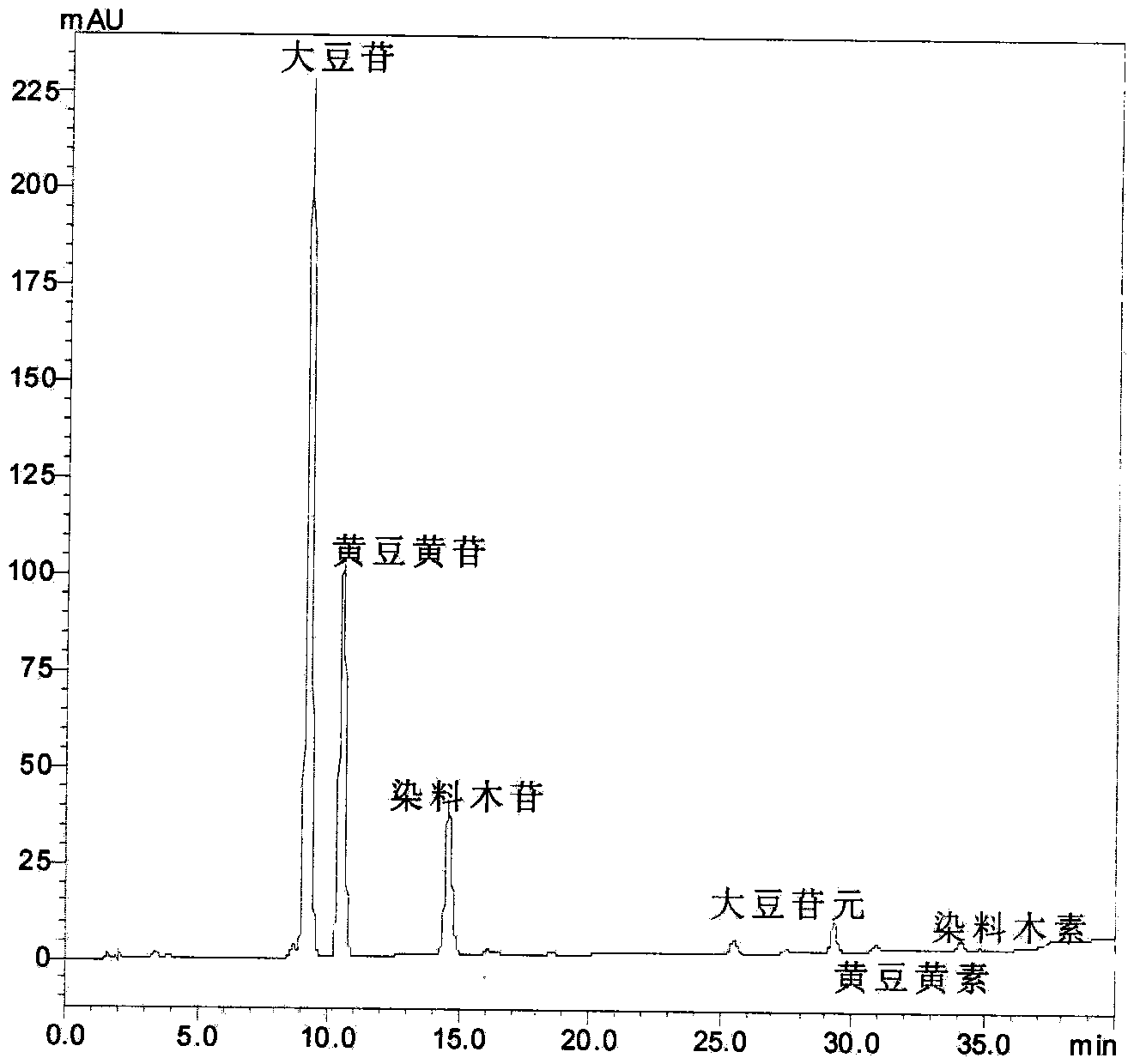
[0024] Take 50mg of 20.5% soy isoflavone glycosides into a 50mL round-bottomed flask, add 2.5mol·L -1 10 mL of citric acid aqueous solution, ultrasonic water bath for 1 hour, ultrasonic frequency 45KHz, water bath temperature 75°C. The reaction liquid was transferred to an autoclave, the reaction temperature was 130° C., and the reaction time was 3.5 hours. After the reaction, the reaction liquid was cooled.
[0025] Other operations are the same as in Example 1. Take a certain amount of the reaction solution and neutralize it with saturated sodium carbonate aqueous solution to pH=7, extract it with ethyl acetate, take the ester layer, spot the sample on the silica gel plate, and put the developing agent (ethyl acetate: Develop in chloroform:methanol=5:5:2) and observe the degree of hydrolysis under ultraviolet light. At the same time, another certain amount of reaction solution was diluted to 100 ppm with methanol, and the content of glycoside and aglycon in the reaction solutio...
Embodiment 3
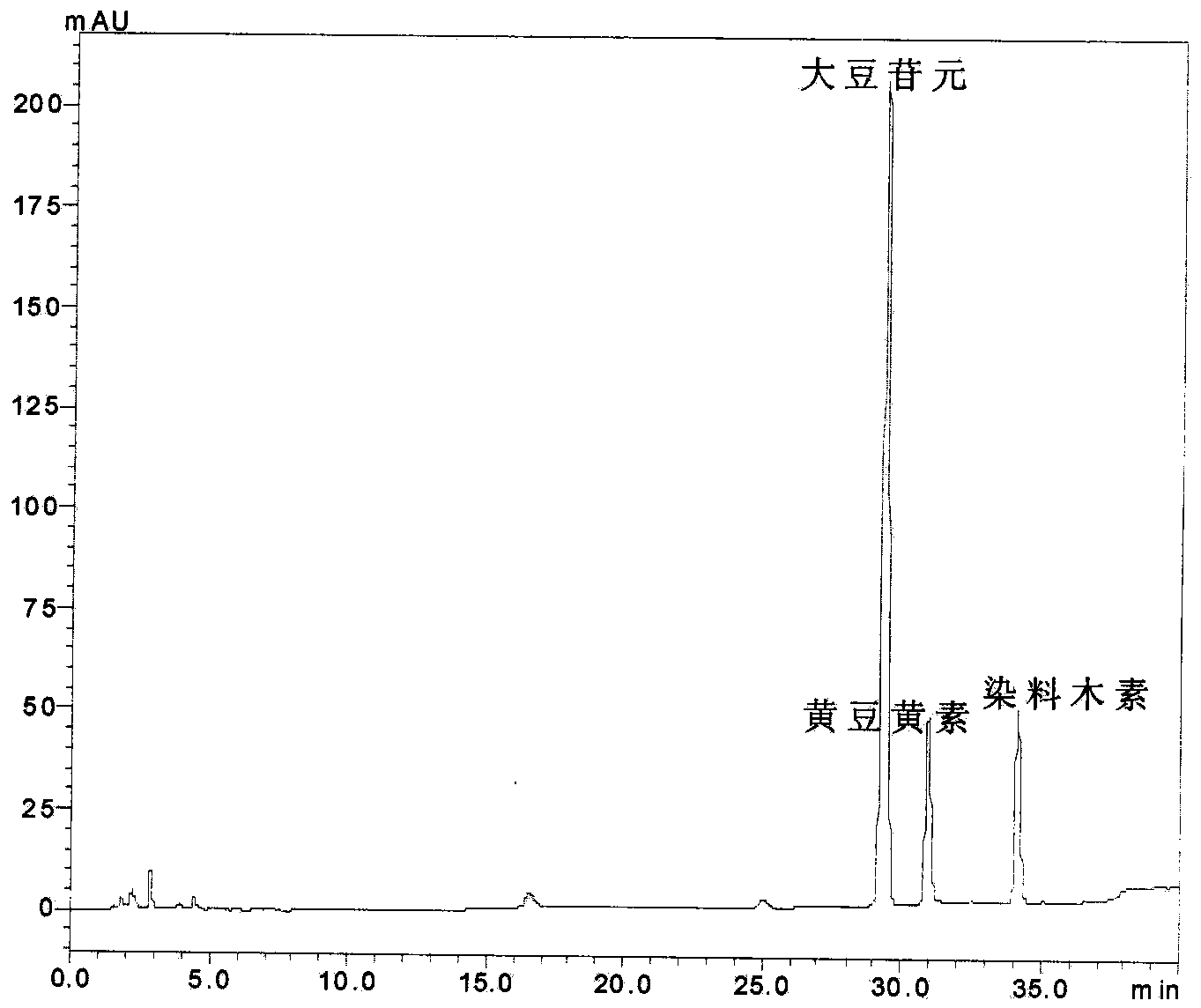
[0027] Take 50mg of 40.0% soybean isoflavone glycosides and place them in a 50mL round-bottom flask, add 3.5mol·L -1 10ml of citric acid aqueous solution, ultrasonic water bath for 1 hour, ultrasonic frequency 45KHz, water bath temperature 75℃. The reaction liquid was transferred to the autoclave, the reaction temperature was 120° C., and the reaction time was 3 hours. After the reaction, the reaction liquid was cooled.
[0028] Other operations are the same as in Example 1. Take a certain amount of the reaction solution and neutralize it with saturated sodium carbonate aqueous solution to pH=7, extract it with ethyl acetate, take the ester layer, spot the sample on the silica gel plate, and put the developing agent (ethyl acetate: Develop in chloroform:methanol=5:5:2) and observe the degree of hydrolysis under ultraviolet light. At the same time, another certain amount of reaction solution was diluted to 100 ppm with methanol, and the glycoside and aglycon content of the reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com