Tanshinone class I derivant and synthesizing method and application thereof
A technology of tanshinone and derivatives, which is applied in the preparation of anti-tumor tanshinone I derivatives and its preparation field, and can solve the problems of difficulty in making dosage forms, low yield, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
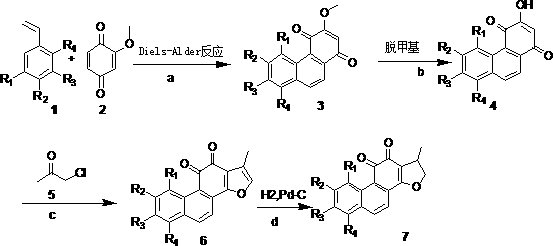
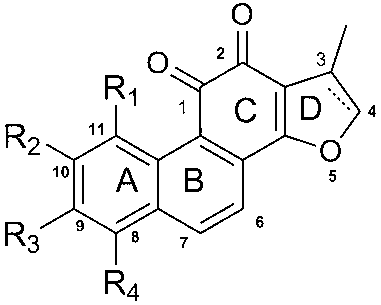
[0072] Example 1 3-methoxy-8-methyl-1,4-phenanthrenedione
[0073] In the autoclave, add 2-methoxy-1,4-p-benzoquinone (0.5mmol) and o-methylstyrene (3mmol) in sequence, and add an appropriate amount of toluene to mix the two evenly, seal it, and do not apply external pressure , heated to 200°C, and stirred for 3h. TLC detects that the reaction is complete. The reaction liquid was cooled and separated by silica gel column, and the developing solvent was dichloromethane:petroleum ether (2:5). 59 mg of orange-red solid was obtained, and the yield was 65%. 1H NMR ( CDCl3, 400 Hz, TMS ): δ2.744 (s,3H), 3.942 (s,3H), 6.147(s,1H), 7.474(d,1H,J=6.8Hz), 7.628(t, 1H, J=16Hz), 8.224(d,1H, J=8.4Hz), 8.398(d,1H, J=9.2Hz), 9.401(d,1H, J=8.8Hz).
Embodiment 2
[0075] Example 2 3,6,7-trimethoxy-1,4-phenanthrenedione
[0076] The substrates were the corresponding styrene derivatives and 2-methoxy-1,4-p-benzoquinone to obtain 107.4 mg of an orange-red solid with a yield of 60%. 1H NMR ( CDCl3, 400 Hz, TMS ): δ3.920(s,3H,OCH3), 4.051(s,3H,OCH3), 4.110(s,3H,OCH3), 6.102(s,1H,AR-H) , 7.121(s,1H, AR-H), 7.952(d,1H, J=8.8Hz, AR-H), 8.11(d,1H, J=8.4Hz, AR-H), 9.145(s,1H, AR-H).
Embodiment 3
[0077] Example 3 3,6,-Dimethoxy-1,4-phenanthrenedione
[0078] The substrates were the corresponding styrene derivatives and 2-methoxy-1,4-p-benzoquinone to obtain 120.5 mg of an orange-red solid with a yield of 65%. 1 H NMR ( CDCl 3 , 400 Hz, TMS ): δ3.940(s,3H), 4.002(s,3H), 6.138(s,1H), 7.290(dd,1H,J=11.6Hz), 7.788(d,1H, J= 9.2Hz), 8.064(d,1H, J=8.0Hz), 8.116(d,1H, J=8.4Hz), 9.056 (d,1H, J=2.4Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com