Method for measuring ion strength of substance
A technology of ionic strength and material, applied in the field of chemical engineering, can solve the problems of large ionic strength error and long time, and achieve the effect of simple operation, avoiding range limitation, and economical operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
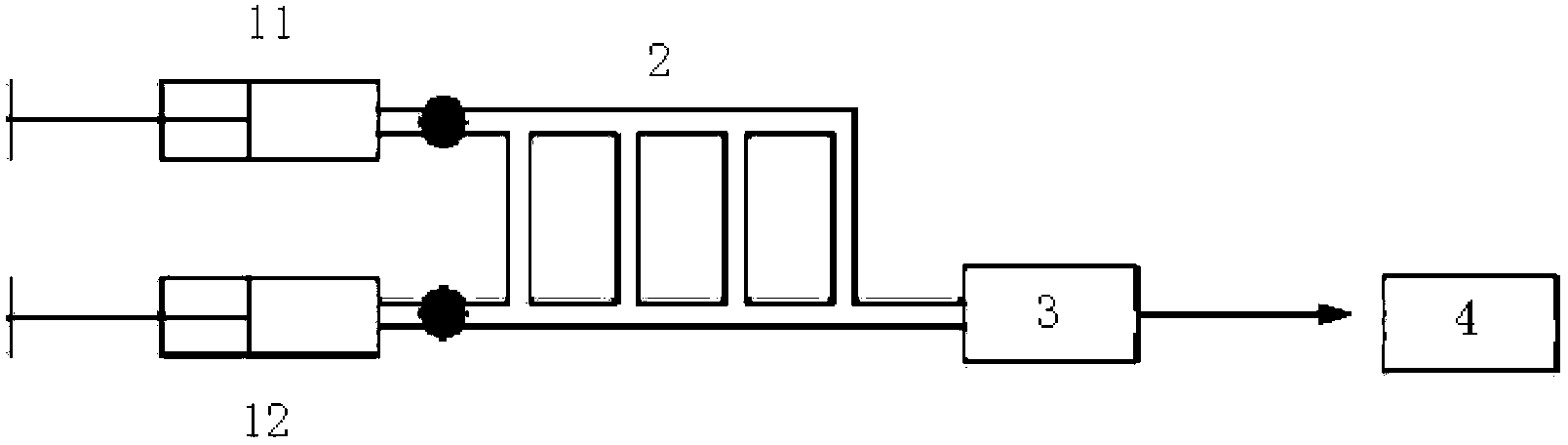
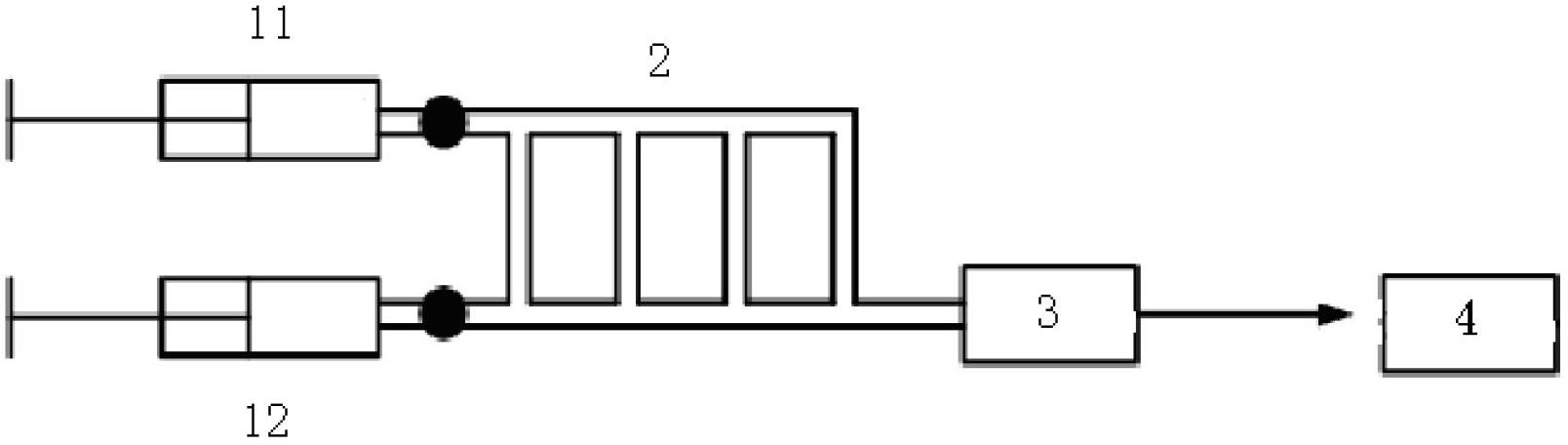
[0022] According to a typical embodiment of the present invention, the method for measuring the ionic strength of a substance comprises the following steps: preparing the substance solution to be tested as a microdispersed droplet emulsion by a liquid-liquid microdispersion method; sending the microdispersed droplet emulsion into an electric field for determination electrophoretic mobility; and obtaining the ionic strength of the microdispersed droplet emulsion according to the value of the electrophoretic mobility.
[0023] The invention prepares the microdispersed droplet emulsion through the liquid-liquid microdispersion method, measures the electrophoretic mobility of the microdispersed droplet emulsion under the action of an electric field, and obtains the ionic strength of the microdispersed droplet emulsion according to the numerical value of the electrophoretic mobility. The invention provides an accurate and effective method for measuring the ionic strength of a soluti...
Embodiment 1
[0040] (1) Establish electrophoretic mobility-ionic strength linear regression equation
[0041] Preparation of standard potassium chloride solution: prepare a series of potassium chloride standard solutions with molar concentrations of 0.1mmol / L, 0.25mmol / L, 0.50mmol / L, 0.75mmol / L, and 1.00mmol / L, according to The ionic strength is equal to half of the sum of the items obtained by multiplying the molar concentration of each ion in the solution by the square of the ion valence, and calculates the ionic strength of the reference potassium chloride solution. The ionic strength of the reference potassium chloride solution and its molar concentration Consistent, respectively: 0.1mmol / L, 0.25mmol / L, 0.50mmol / L, 0.75mmol / L, 1.00mmol / L.
[0042] The above-mentioned reference potassium chloride solution is formulated into a reference microdispersion droplet emulsion: dodecane (molar concentration is 5 × 10 -4 mol / L) and methanol according to the mass percentage of 1:1000 to obtain a ...
Embodiment 2
[0052] (1) Establish electrophoretic mobility-ionic strength linear regression equation
[0053] Preparation of standard potassium nitrate solution: configure a series of potassium nitrate standard solutions with molar concentrations of 0.1mol / L, 0.25mol / L, 0.50mol / L, 0.75mol / L, and 1.00mol / L, according to the ionic strength equal to each The molar concentration of the ion is multiplied by the half of the sum of the items obtained by the square of the ion valence, and the ionic strength of the reference potassium nitrate solution is calculated. The ionic strength of the reference potassium nitrate solution is consistent with its molar concentration, which is respectively: 0.1mmol / L, 0.25mmol / L, 0.50mmol / L, 0.75mmol / L, 1.00mmol / L.
[0054] The above-mentioned reference potassium nitrate solution is formulated into a reference microdispersion droplet emulsion: hexadecane (molar concentration is 70×10 -4 mol / L) and acetone according to the mass percentage of 6:1000 to obtain a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com