Functionalized united imidazole derivative and synthesizing method thereof
A technology of functional grouping and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effect of simple and economical operation, easy post-processing, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of 1,1'-bis(amidomethyl)-2,2'-biimidazole
[0053] Add 5g (0.125mol) of solid sodium hydroxide to 13.4g (0.1mol) of 2,2′-biimidazole in DMF (100mL) suspension, and heat to 80°C under stirring. After stirring for 30 minutes, the solution turns dark green , dropwise added a DMF solution of 2-chloroacetamide (0.1mol), the concentration was 0.2mol / 100mL, and the dropwise addition was completed in 15 minutes. After 30 minutes of reaction, 4 g (0.1mol) of sodium hydroxide solid was added, and after another 30 minutes, dropwise Add the same part of the DMF solution of 2-chloroacetamide, the concentration is 0.2mol / 100mL, after the dropwise addition, the reaction is kept at 80°C for 6 hours. Naturally cooled to room temperature, a light yellow solid precipitated, which was recrystallized twice with ethanol to obtain a white solid product.
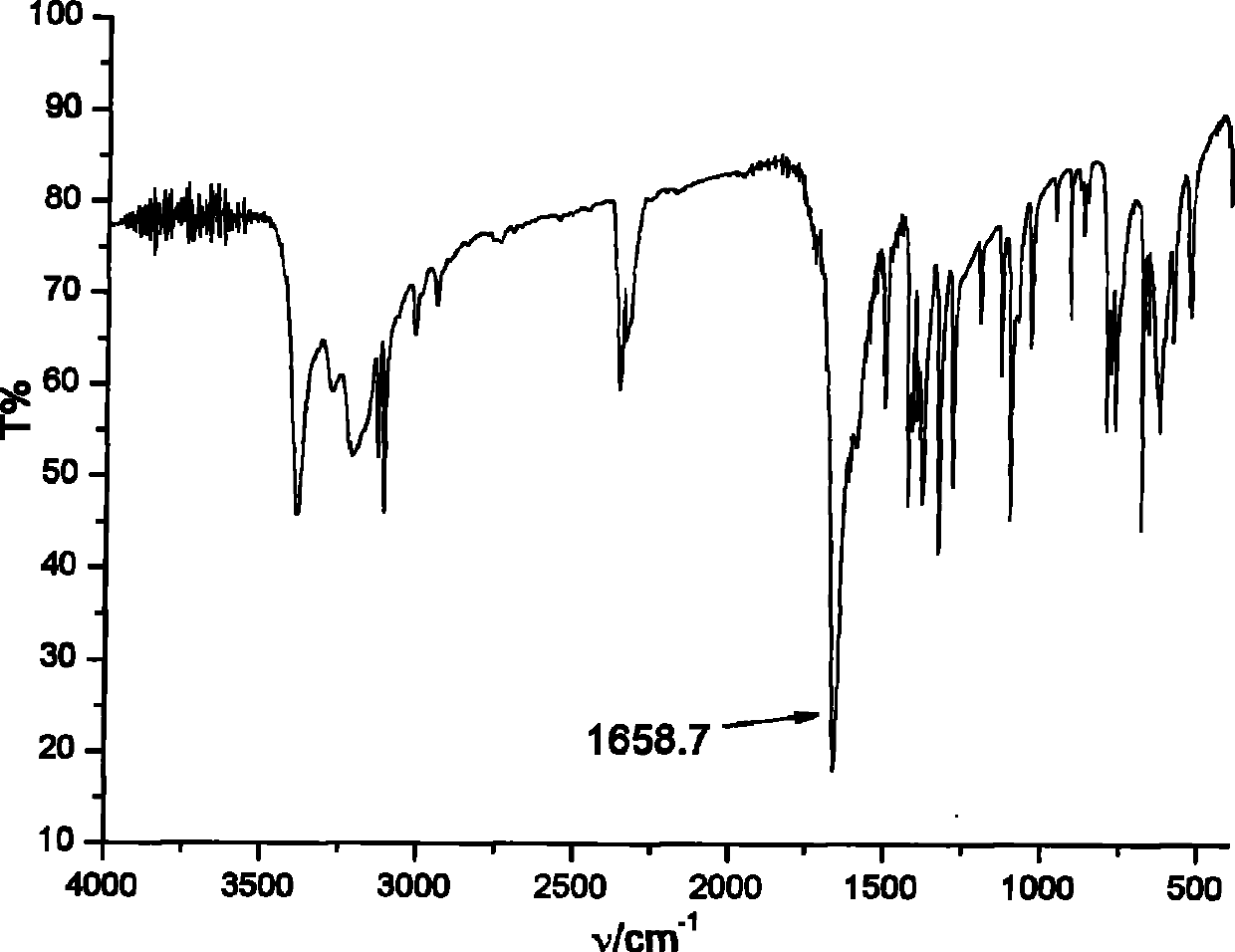
[0054] Infrared: (KBr, cm -1 ): 3363, 1658, 1334, 1101, 686. The infrared spectrum of the product is attached figure 1 .
Embodiment 2
[0056] Preparation of 1,1'-bis(2-amidoethyl)-2,2'-biimidazole
[0057] 2,2'-Bimidazole 13.4g (0.1mol) in DMF (100mL) suspension, add 0.8g (0.02mol) sodium hydroxide 10mL aqueous solution, and heat to 100°C while stirring. Add a solution of 14.2g (0.2mol) of acrylamide in DMF (50mL) dropwise, the concentration is 0.4mol / 100mL, biimidazole is slowly dissolved to obtain a clear solution, the color of the solution is from colorless, green to orange. After refluxing for six hours, cool naturally, distill DMF under reduced pressure to the remaining 1 / 4 volume, and solid precipitates, then put it in the refrigerator to freeze for 10 hours, filter while cold, wash with 10mL ether for 3 times, and dry in vacuum to obtain a white solid 20.57 g, Yield: 74.3%.
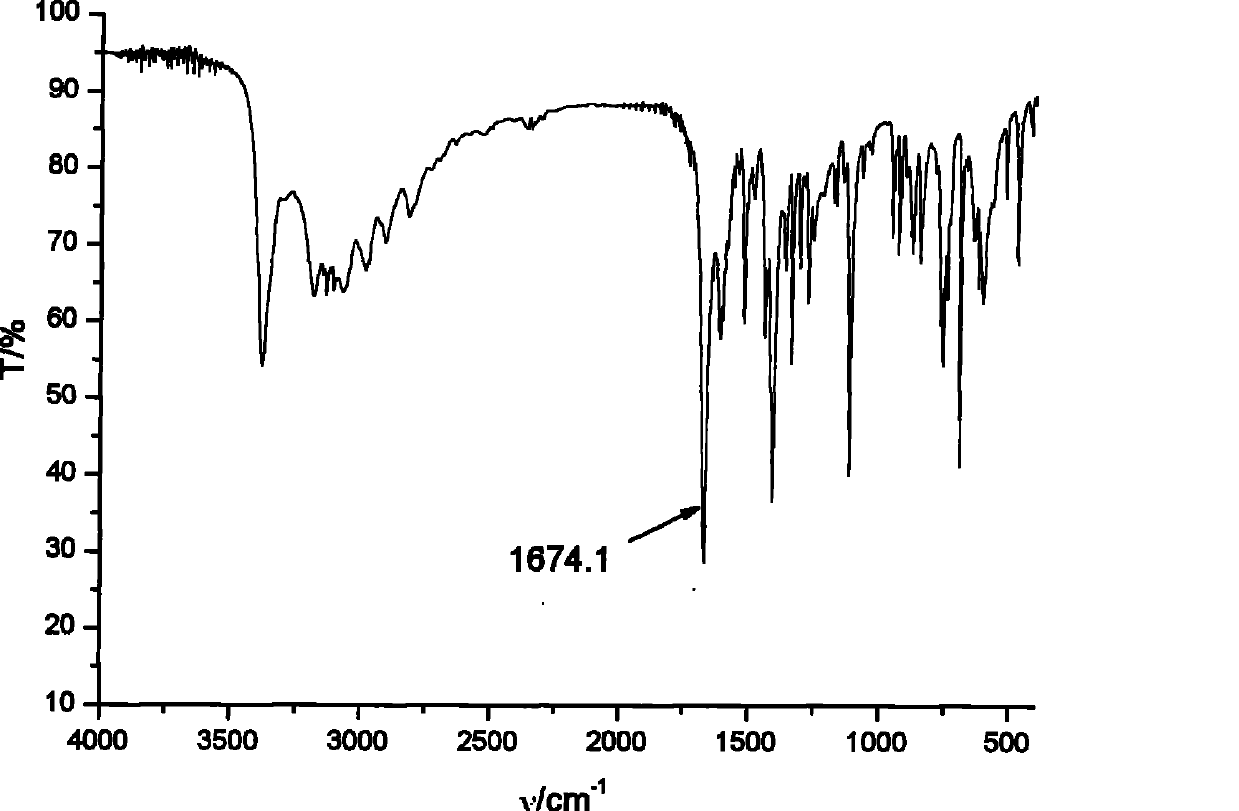
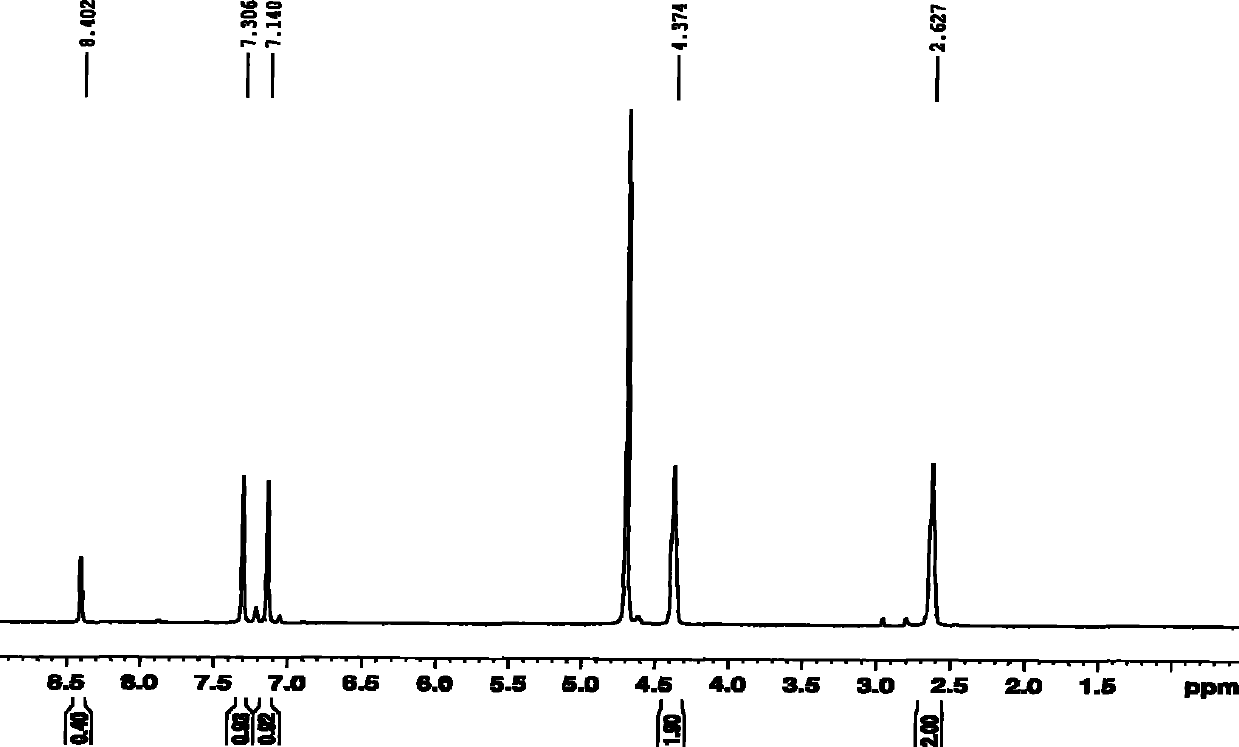
[0058] IR: (KBr, cm -1 ): 3388, 1674, 1409, 1267, 769; 1 H NMR: (400MHz, D 2 O, 25°C, TMS, ppm) δ: 8.402 (s, 4H), 7.306 (s, 2H), 7.140 (s, 2H), 4.374 (s, 4H), 2.627 (s, 4H). 13 C NMR: (100MHZ, D 2 O, 25°C, TMS, ppm) δ: 171.5...
Embodiment 3
[0061] Preparation of 1,1'-bis(carboxymethyl)-2,2'-biimidazole
[0062] Take 1.28g (6.04mmol) of 1,1'-bis(cyanomethyl)-2,2'-biimidazole and add it to 3mL (54mmol) of 98% concentrated sulfuric acid in water, heat to 100°C, and reflux for 10 hours , after cooling, the pH was adjusted to 1 with 1M aqueous sodium hydroxide solution, and a solid precipitated out, filtered, washed twice with a small amount of water, and dried in vacuo to obtain 0.862 g of a white solid product, yield: 62%.
[0063] IR: (KBr, cm -1 ): 3145, 1732, 1348, 1245, 767. 1 H NMR: (400MHz, D 2 O, 25°C, TMS, ppm) δ: 12.921 (s, 2H), 7.280 (d, J = 0.8 Hz, 2H), 6.997 (d, J = 0.8 Hz, 2H), 5.309 (s, 4H). 13 C NMR: (100MHz, D 2 O, 25°C, TMS, ppm) δ: 169.78, 137.84, 127.02, 123.41, 49.00.
[0064] The infrared spectrum, proton nuclear magnetic resonance spectrum and carbon spectrum of the product are shown in the appendix Figure 5 , 6, 7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com