Compositions and methods for treating hepatitis b virus infection
A technology of hepatitis B virus and composition, applied in the directions of antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Implementation Example 1. Formulation
[0148] Although lamivudine and adefovir dipivoxil can be co-administered as bulk drugs, they are preferably administered via a pharmaceutical composition of these compounds that is convenient for patient use. Table 1 lists the dosage examples of each of lamivudine and adefovir dipivoxil used to prepare pharmaceutical composition preparations.
[0149] Table 1. Examples of respective unit doses used in the preparation of lamivudine and adefovir dipivoxil pharmaceutical compositions
[0150]
[0151] Prepare examples of each lamivudine dose, such as 405, 460, 510, 550, 600 and 900 mg / unit dose, and each example of adefovir dipivoxil dose, such as 3, 5, 8 and 10 mg / unit dose, Pharmaceutical composition preparations, 24 kinds of composition preparations with different dosage strengths can be obtained.
[0152] Each dosage strength listed in Table 1 can be prepared into conventional pharmaceutical preparations with appropriate pharmaceutical...
Embodiment 2
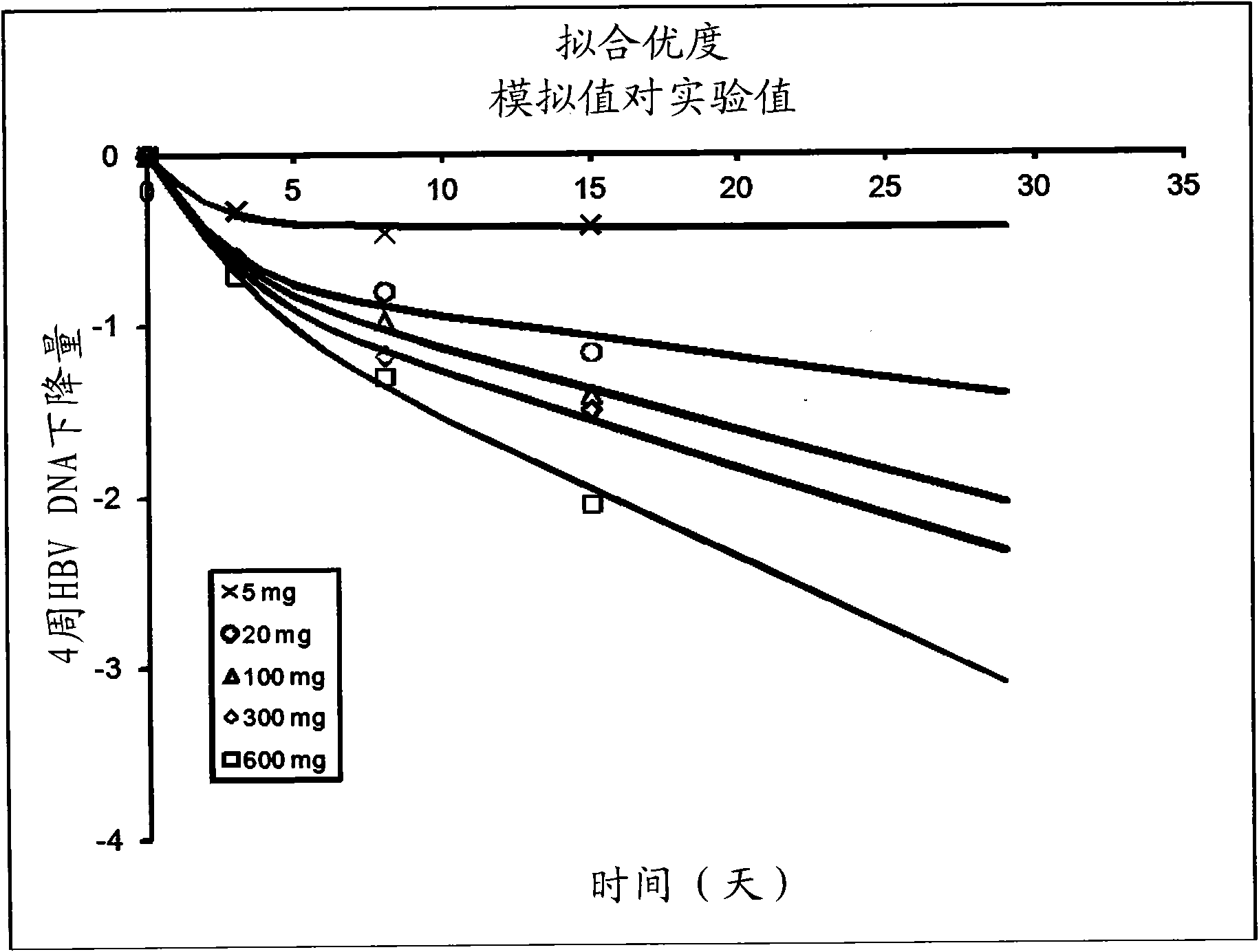
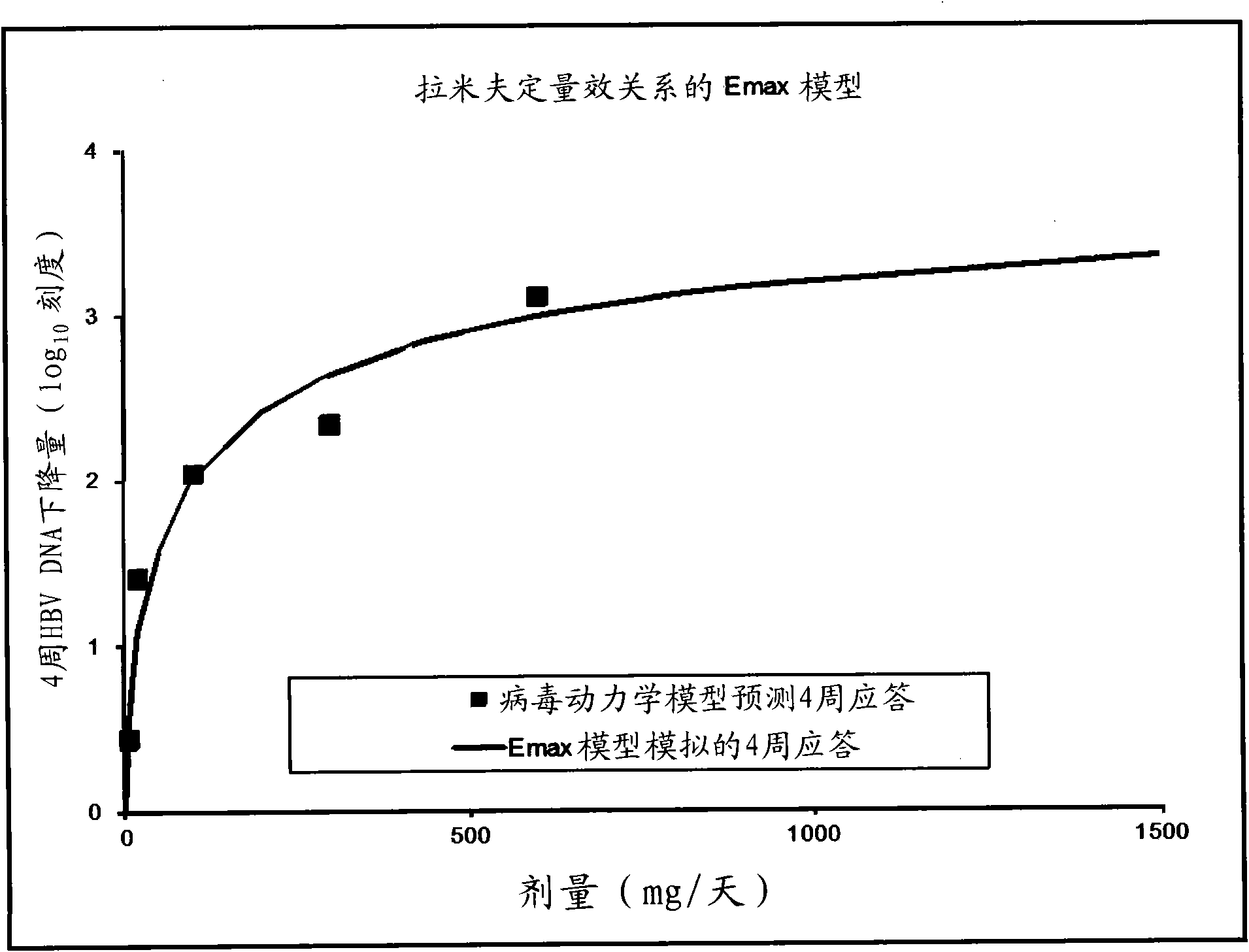
[0156] Implementation example 2. Virus kinetic analysis and Emax model
[0157] HBV DNA data
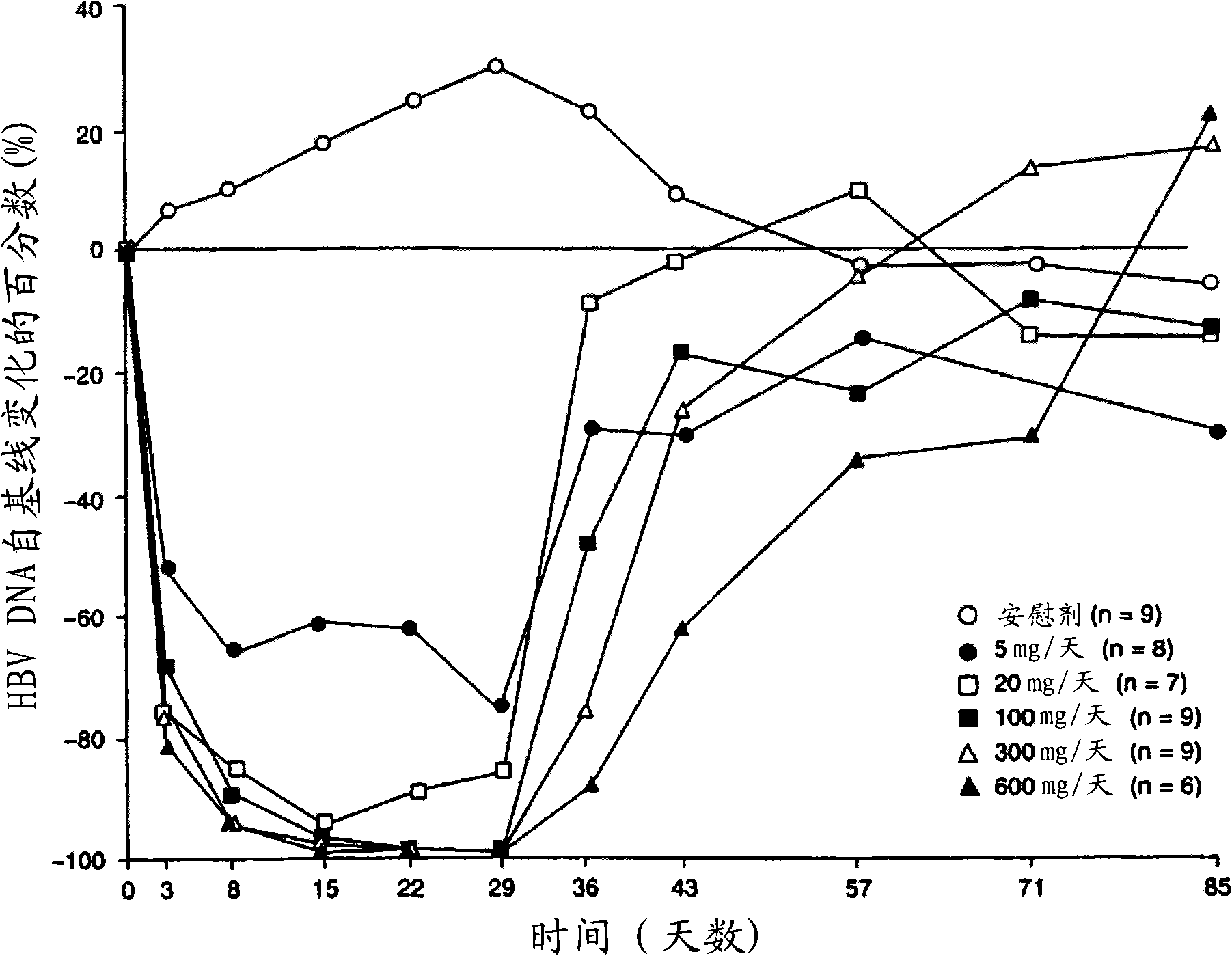
[0158] The HBV DNA data used in the unique viral kinetic analysis during the lamivudine treatment disclosed here is derived from the published lamivudine quantitative-effect curve ( figure 1 ) (Johnson et al. Clinical pharmacokinetics of lamivudine, Clin Pharmacokinet 1999, 36(1): 41-66). figure 1 The data points shown to 29 days were obtained by scanning and digitizing. Given that the HBVDNA value is too close to the off-line of quantitative methods, the data on the 22nd and 29th days of the high-dose group is not reliable. Therefore, virus kinetic analysis only used data up to 15 days.
[0159] Virus kinetic analysis
[0160] Convert the digitized data into the percentage change from the baseline (V t -V 0 ) / V 0 It is further expressed as V t / V 0 ; Where V 0 And Vt are the HBV DNA values at baseline and t time after the start of treatment, respectively. Use the following virus kinet...
Embodiment 3
[0180] Implementation example 3. Clinical data
[0181] A preliminary observational study initiated by doctors to evaluate the clinical efficacy of the optimal dose of lamivudine combined with low-dose adefovir dipivoxil in HBeAg-positive chronic hepatitis B patients is ongoing. The results of the interim case report of 6 months of treatment are summarized below.
[0182] TMS is a 46-year-old male of Chinese descent. When he saw a doctor on April 11, 2010, he stated that he had a history of chronic hepatitis B (CHB) for several years and had recently felt weakness and decreased appetite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com