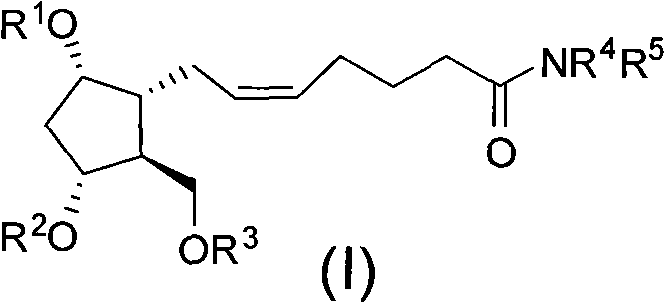
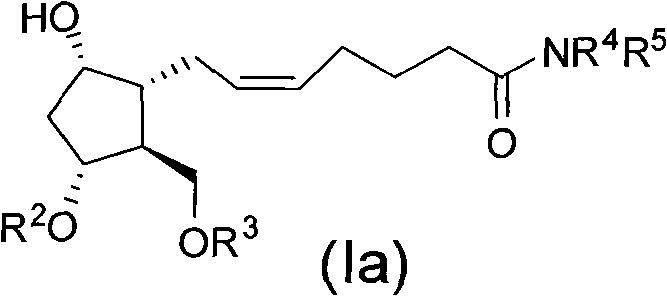
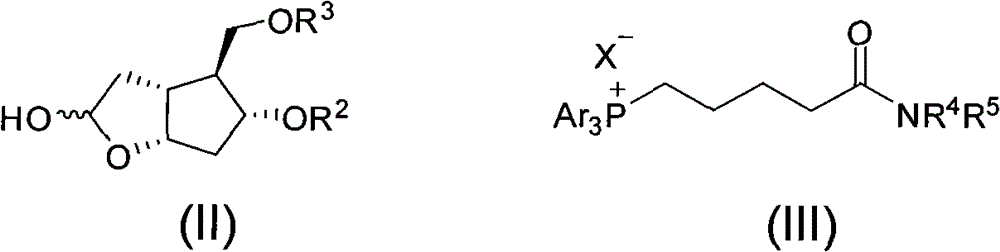Intermediate for synthesizing prostaglandin drugs and its preparation method
A technology for prostaglandins and intermediates, which is applied in the field of intermediates for the synthesis of prostaglandins, and can solve the problems that prostaglandins cannot meet the needs of clinical medication and their content is low
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: Preparation of compound Iaa
[0032]
[0033] Compound Iaa is synthesized as follows:
[0034]
[0035] step 1):
[0036] Under a nitrogen atmosphere, triethylamine (2.5 mL) was added to a solution of compound IV (1.0 g, purchased from Shanghai Julong Pharmaceutical Research and Development Co., Ltd.) in dichloromethane (50 mL). The reaction system was cooled to -78°C, and a solution of triisopropylsilyl triflate (2.9 g) in dichloromethane (10 mL) was added. After two hours, the cooling bath was removed, and the reaction system was stirred at 20°C for 30 minutes. The reaction system was concentrated under reduced pressure, and the residue was purified by column chromatography to obtain compound Vaa.
[0037] 1 HNMR (400MHz, CDCl 3 ): δ4.94-4.89(m, 1H), 4.18-4.11(m, 1H), 3.84(dd, J=9.6, 5.2Hz, 1H), 3.69(dd, J=10.4, 7.2Hz, 1H), 2.79(dd, J=18, 10.8Hz, 1H), 2.63-2.60(m, 1H), 2.53-2.45(m, 2H), 2.70(s, 1H), 2.05-1.97(m, 2H), 1.14- 1.01(m, 21H)
[0...
Embodiment 2
[0044] Embodiment 2: preparation compound Iab
[0045]
[0046] Using a method similar to the synthesis of compound Iaa, compound Iab was synthesized.
[0047] 1 H-NMR (400MHz, CDCl 3 ( m, 4H), 2.39~2.33(m, 1H), 2.19~2.00(m, 5H), 1.89~1.85(m, 3H), 1.73~1.58(m, 3H), 1.13~1.09(m, 3H), 0.89~0.85(m, 9H), 0.07~0.02(m, 6H)
Embodiment 3
[0048] Embodiment 3: preparation compound Iac
[0049]
[0050] Compound lac was synthesized using a method similar to the synthesis of compound Iaa.
[0051] 1 H-NMR (400MHz, CDCl 3 )δ5.98~5.96(m, 1H), 5.41~5.34(m, 2H), 4.14~4.10(m, 2H), 3.76~3.72(m, 1H), 3.49~3.45(m, 1H), 3.34~ 3.28(m, 2H), 3.22~3.17(m, 2H), 2.40~2.32(m, 1H), 2.18~2.10(m, 4H), 2.05~1.99(m, 1H), 1.85~1.83(br, 3H ), 1.71~1.58(m, 3H), 1.48~1.40(m, 2H), 1.35~1.28(m, 2H), 0.90~0.85(m, 3H), 0.84~0.83(m, 9H), 0.05~0.01 (m, 6H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com