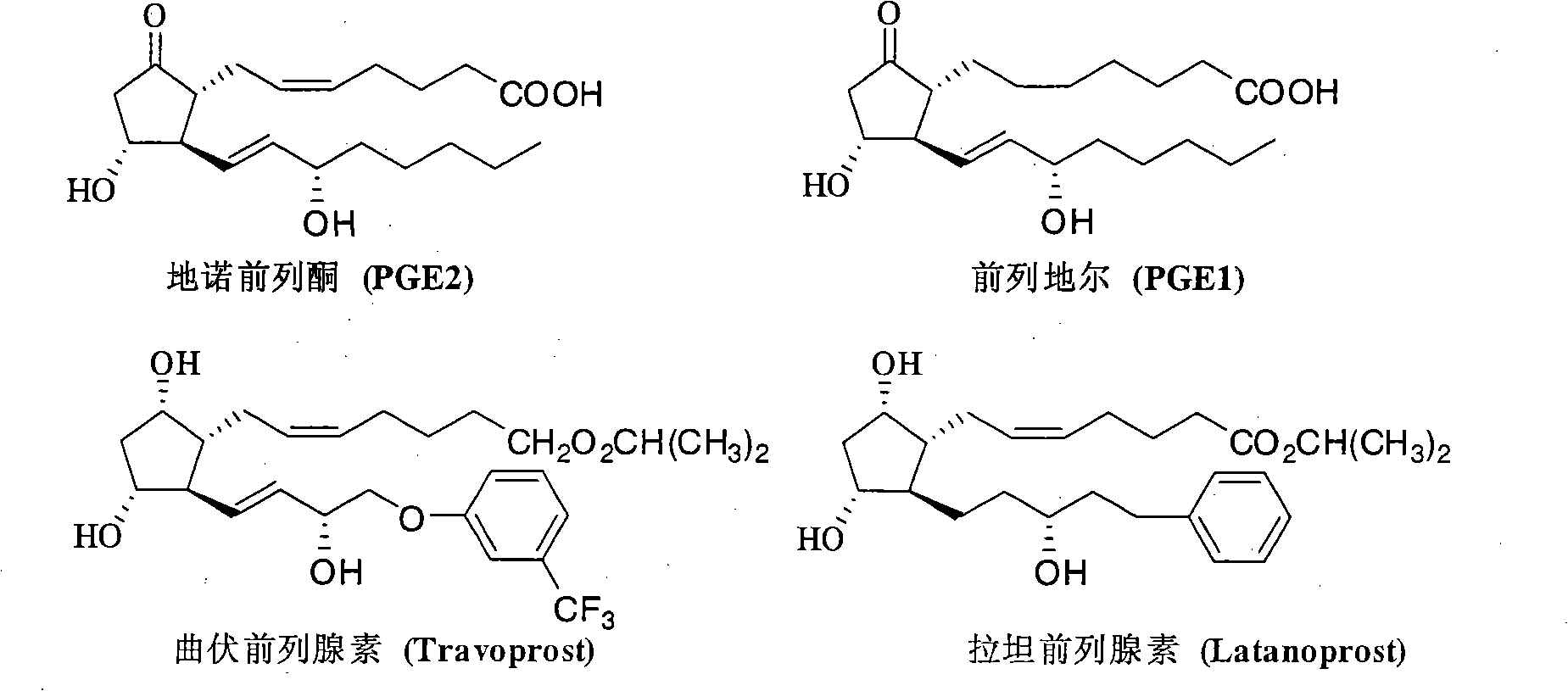
New technology for synthesizing Corey lactonic acid by one-pan boiling method
A technology, pot cooking technology, applied in the new technology field of one-pot cooking to synthesize Corey lactone, can solve the problems of unsafe storage and transportation, low yield, high risk, etc., achieve room temperature storage or transportation safety, and reduce types and quantity, the effect of avoiding product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
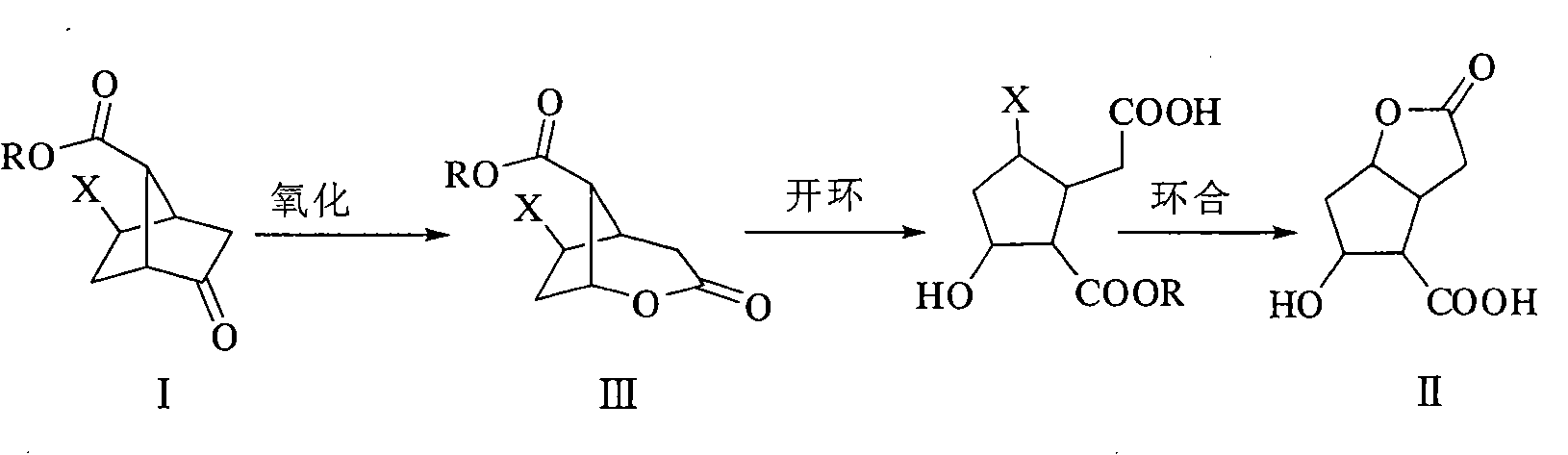
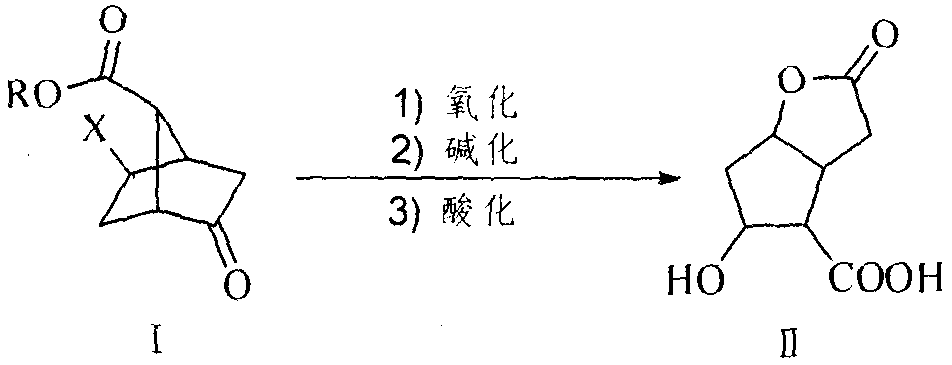
[0039] Preparation of methyl 6-chloro-3-oxo-2-oxabicyclo[3.2.1]octane-8-carboxylate
[0040] Add 2-halo-5-oxobicyclo[2.2.1]heptane-7-carboxylic acid methyl ester 5g, 4-butylammonium bromide 20mg, CH 2 Cl 2 50ml, water 50ml, then add oxone 22.5g in batches, stir and react for 48 hours, TLC plate detects that there is no obvious raw material residue, filter with suction, filter cake with CH 2 Cl 2 10ml×2 washing, the filtrate was washed with NaHSO 3 The saturated solution was washed with 30ml×2, the organic phase was separated, dried over anhydrous sodium sulfate, filtered with suction, evaporated to dryness, and recrystallized with water to obtain white crystals.
Embodiment 2
[0042] Preparation of 6-chloro-3-oxo-2-oxabicyclo[3.2.1]octane-8-carboxylic acid
[0043] Add 5g of 2-halo-5-oxobicyclo[2.2.1]heptane-7-carboxylic acid, 50ml of acetone-water mixed solution in turn to the reaction bottle, then add 22.5g of oxone in batches, stir the reaction overnight, TLC plate No obvious raw material residue was detected, filtered with suction, the filter cake was washed with acetone 10ml×2, the filtrate was evaporated under reduced pressure to remove methanol, extracted with ethyl acetate 100ml×3, the combined organic layer was washed with saturated brine 100ml×2, and the organic The layer was dried overnight with anhydrous sodium sulfate, filtered with suction, evaporated to dryness, and recrystallized from acetone-ether to obtain white crystals.
Embodiment 3
[0045] Preparation of 5-Hydroxy-hexahydro-2-oxo-2H-cyclopenta[b]furan-4-carboxylic acid
[0046]Add 80g of 2-chloro-5-oxobicyclo[2.2.1]heptane-7-carboxylic acid and 500ml of methanol in sequence to the reaction flask, then gradually add 270g of OXONE, and stir the reaction at room temperature. No obvious raw material residue is detected by TLC. Add sodium bisulfite to quench the reaction, dropwise add 10% NaOH to alkalinize, continue the reaction, add concentrated hydrochloric acid to adjust the pH value to acidity, concentrate the reaction solution under reduced pressure, extract with THF, separate the organic layer, dry over anhydrous sodium sulfate, and extract Evaporate by filtration to obtain a white solid, and wash with ether to obtain the target product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com